Hydrolysis
A similar method is suitable if you need to clean a complex-shaped tool that consists of a large number of parts. It is pointless to skin or brush such elements, so hydrolysis remains. Its principle is to purify almost at the molecular level
In the process of hydrolysis, hydrogen is released, it is able to quickly ignite, therefore, during work, it is important that the room is well ventilated
- A container for water made of a dielectric material (such as plastic).
- A piece of stainless steel (for example, a piece of a new stainless steel pipe cut in half).

Screenshot
- Sewer pipe cleaner or caustic soda.
- Constant current source. For example, you can use a car charger or a computer power adapter.
Healthy! Some don't use stainless steel. Instead, the hydrolysis process is carried out in a metal bucket to which a baking soda solution is added. This option also works. But it must be borne in mind that during hydrolysis, a strong heating of the liquid occurs. In a plastic bucket, it reaches 50 degrees, in a metal container, the heating will be more.
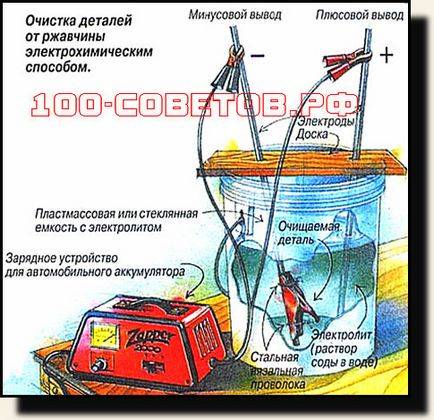
The rust removal process itself is as follows:
- We put the stainless steel in a plastic bucket. The stainless steel should be located along the wall of the container, thereby creating a kind of screen.

Screenshot
- In order to get a good contact, it is necessary to first clean a small area on the instrument (a "crocodile" will be installed on it later).
- We lower the tool into the bucket. At the same time, he must not be allowed to touch the screen. Therefore, the part must be suspended (for example, using a clamp and a small plastic pipe, which we will put on the edge of the bucket).

Screenshot
- We fill the bucket with water so that it covers the tool completely.
- Add pipe cleaning fluid (2-3 caps for 1 bucket of water) and mix well with water.
- It is necessary to connect “+” (red wire) to the stainless steel, and “minus” (red-black wire) to the part to be cleaned.

Screenshot
- We connect the charger in order to receive a voltage of about 9-12 volts at the output. The stronger the current is applied, the faster the cleaning will take place.
- The liquid will foam a little. This is a normal hydrolysis process.
- We leave the part in this state for the night.
After that, it remains to remove the tool and clean it from peeling rust. For simple tools, this method is laborious. But for large elements (for example, a rusted vise) it is perfect. Also, this method can be used to clean car parts. For pliers, screws, bolts and more, you should choose the simpler cleaning methods described above. The best options are citric acid and vinegar.
How to prolong the effect?
The protective zinc layer that forms on the surface can protect the metal from corrosion for 4-6 weeks. To increase this gap, you can varnish the treated area - this way it will be completely protected from the aggressive effects of the external environment. But this method has disadvantages: on visible elements (doors, hood), the transitions over the varnish can be very noticeable.
Therefore, it is better to carry out preventive treatment with an acid composition every 1-1.5 months in order to avoid erasing the protective layer. In addition, you should keep the car clean and dry, after driving in snowy or rainy weather, be sure to dry the bottom.
For maximum effect, it is advisable to equip the garage with good temperature control and ventilation systems.To avoid cracks and paint peeling, do not wash the machine with hot water during cold seasons. Lamination of the body can also help - the polymer film will reliably protect against temperature extremes and environmental influences.
How and how to remove rust from metal
Removing traces of corrosion from various objects has its own specifics, which must be taken into account.
From the car body
Rust often forms on the car body. The following remedies are best for removing it:
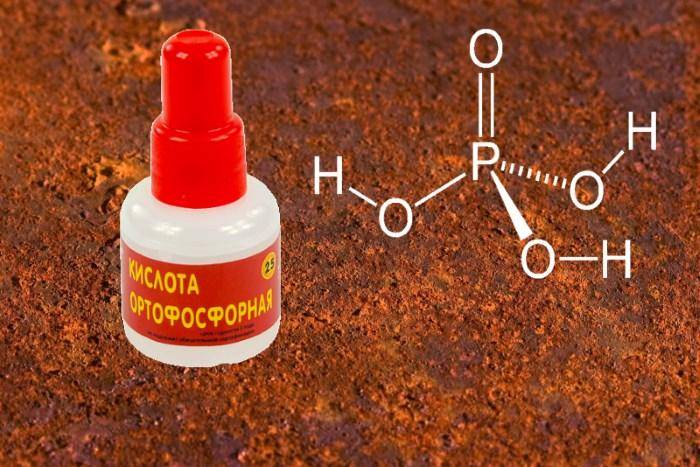
- Orthophosphoric acid. Its solution is applied to a sponge, which is used to wipe the car body.
- Zinc. Mixtures are made from it, allowing to get rid of contamination after the first treatment.
From the water tap
The best remedy for rust on metal, especially for cleaning metal enameled surfaces, is the Adrilan product, produced for washing household appliances. Since it is very concentrated, it is diluted in warm water before use.
An effective cleaning agent, and not only against rust, but also against any contamination.
From the bike
Rust stains that appear on the bike frame over time can be easily removed with citric acid. At the same time, before removing rust from the metal, the surface must be degreased, and then rinsed and dried thoroughly.
From skates
If the skates are stored for a long time in high humidity conditions, rusty deposits begin to form on them. You can remove it with a mixture of baking soda and lemon juice. For this, the components are mixed until a mushy substance is obtained, which must be applied to the contaminated surface for an hour and a half. After that, the skates must be rinsed and dried.
Effective folk remedies will help to eliminate rusty spots from skates.
From a horseshoe
Old rusty horseshoe can be cleaned with oxalic acid. To create a solution, it is necessary to mix acid and boiled water in a ratio of 1 to 12. A horseshoe is placed in the resulting liquid for forty minutes, after which it is washed with running water.
With tools
Metal tools that are rarely used corrode over time. You can clean them of rusty deposits with a vinegar solution. To prepare it, you need to mix white vinegar with warm water in equal proportions. The resulting liquid is applied to contaminated instruments, after which they can be easily cleaned with a metal brush or sponge.
Pour the vinegar into a container to fit the spoiled item. Immerse the product in the container with the product.
After cleaning, polished metal tools can be treated with a solution of gasoline and wax or paraffin to protect them from further corrosion. To obtain it, paraffin or wax must be added to gasoline heated in a water bath.
With a nut
You can clean the nuts from corrosive deposits with a vinegar solution. To do this, 100 ml of white vinegar is diluted in a bucket of water, after which all rusted nuts are placed in a container for three to four hours. After this time, the nuts are rinsed with clean water and wiped off from rust residues.
Cleaning small household items
All of the above methods can be used to clean small metal objects. The only difference is the ability to place a rusty product entirely in the used product.
Stick to all of the above rules and you will find that rust can be removed quickly and effortlessly.
Industrial methods of prevention
In addition to forced cleaning from rust, there are methods to prevent metal oxidation. They include:
- galvanic treatment;
- cathodic protection;
- application of inert coatings.
In domestic conditions, these methods are problematic to apply due to the lack of appropriate equipment, the complexity of technological processes.
Galvanization
The method involves spraying a thin layer of a substance that is weakly subject to oxidation on a ferrous metal using electrolysis. The nuance of the situation is that as soon as the protection is violated, corrosion begins immediately.

Cathodic protection
A method involving the use of a direct current source, which creates a zone of negative electric potential on the protected surface. It is successfully used on large objects (ships).
Special coatings
Methods of protection with the help of specially applied metal coatings can be no less effective than others. Usually, for their manufacture, substances are used that do not react with condensate or moisture.
Galvanized
Coating with a zinc layer perfectly protects the base metal from oxidation, makes it inert with respect to slightly aggressive media. It is widely used to protect body parts, in the manufacture of hardware, fasteners.
Tinning
The method is based on coating the metal with molten tin solder. The formed layer resists oxidation well and prevents the spread of corrosion.
Chrome plating
It consists in applying a layer of chromium to the units and parts, which is practically not subject to oxidation. Allows you to save money by replacing the production of expensive stainless steel with a production of ferrous metal, followed by chrome plating.
Share link:
A little chemistry to understand the process of rust formation on metal
Rust is a product of iron oxidation. Most often it is a chemical compound Fe₂O₃. This oxide imparts a reddish tint. However, dark blotches can often be observed.

They indicate that the metal has not only trivalent properties, but also bivalent. Therefore, the oxide will be written with the chemical formula FeO.
On the basis of numerous studies, it has been established that Fe₂O₃ prevails. It occurs in 85 ... 88% of cases.
Information for the curious. Iron is smelted from ore. It is customary to denote it as the Fe₃O₄ compound, but this formula does not give a real picture. In nature, the compound occurs in blast furnaces at temperatures above 850 ° C.
Iron in the open air actively interacts with atmospheric oxygen. Therefore, the oxide film is formed rather quickly. In practice, to prevent oxidative processes, the metal surface is protected.
The density of the oxide film can be different. If a steel object has been in the open air for a long time, then the metal may be all permeated with holes consisting of oxide (rust). In fact, it completely loses its properties.
Items that have recently appeared without additional protection are covered only with a thin coating. The thickness of the oxide film is measured in microns. In such a case, the strength of its adhesion is negligible. It can be easily removed from the surface.
Attention! Iron oxide FeO is considered protective. If this compound is present on the surface, then it does not allow the spread of red rust (Fe₂O₃)
Divalent oxidation occurs when the metal is heated above 250 ... 270 ⁰С.
When iron is smelted from ore under the influence of high temperature, the processes of metal reduction with carbon and hydrogen take place:
- 3Fe₂O₃ + CO = 2Fe₃O₄ + CO₂;
- Fe₃O₄ + CO = 3FeO + CO₂;
- FeO + CO = Fe + CO₂;
- 3Fe₂O₃ + H₂ = 2Fe₃O₄ + H₂O;
- Fe₃O₄ + H₂ = 3FeO + H₂O;
- FeO + H₂ = FeO + H₂O
In addition to the formation of pure iron Fe, iron carbide Fe₃C is formed in the blast furnace process. The reaction of its formation occurs at a temperature of 950 ... 1000 ⁰С.
3Fe + 2СО = Fe₃C + CO₂.
The flue gas contains carbon monoxide CO₂ and water vapor H₂O. At a temperature of 1536 ⁰С, iron Fe melts.
The opposite process takes place in nature. In this case, cast iron, due to the high content of iron carbide, oxidizes several times more slowly than steel.
Important! Steel is considered a mechanical connection of iron with carbon, provided that the content is not more than 2.14%. With increasing carbon content, the alloy is cast iron
Methods for chemical treatment of rusted metals
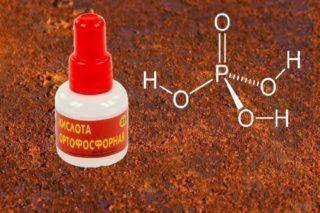 Phosphoric acid - a natural rust converter
Phosphoric acid - a natural rust converter
Any rust remover contains acids. Solutions of mineral compounds - sulfuric, saline or phosphoric - are used against abundant seams. They give quick results, but require precise adherence to processing times. Overexposure will damage undamaged metal. When preparing solutions from these substances with your own hands, they must be diluted to a concentration of no more than 5%.
For light dirt and delicate cleaning, organic acids are used - acetic, oxalic and formic. Their effect can last for a long time, but at the same time the risk of harm to the object to be cleaned is reduced.
Technical fluids are also used to remove rust:
- With gasoline, it is easy to remove only the plaque that has appeared.
- Kerosene penetrates into the smallest cracks and gives a good result when separating parts that have rusted to each other. Prolonged soaking in it can eliminate serious corrosion without affecting healthy metal.
- Phosphoric acid is considered a natural rust converter. It should be applied to the damaged area and after 1-2 minutes rinse with water and wipe dry. Forms a thin, whitish protective film on the surface of pure metal.
Industrial preparations for any rust can be purchased from building departments and auto stores. By the type of action, there are such compositions:
- Liquids. With their help, they wipe slightly rusted objects or watered stronger layers of rust. Contaminated tools and hardware are temporarily placed in a container filled with cleaning liquid.
- Gels and pastes. They are applied to the damaged surface, and after the time specified in the instructions, the remnants of the product, together with rust, are removed with a rag or washed off with water.
- Sprays. They are used to treat hard-to-reach places or to remove fresh traces of rust.
The properties of some industrial cleaners are worth considering in more detail.
Rust remover WD-40
 WD-40 product line includes formulations to remove and prevent rust
WD-40 product line includes formulations to remove and prevent rust
Anti-corrosion products of the American company have long been used to clean and protect any metal surfaces.
The most popular is the drug in the form of an aerosol. It is produced in cans with a long flexible spout, thanks to which it can be sprayed in the most inconvenient places and gaps. The high degree of penetration into the smallest pores allows it to be effectively used to loosen rusted threaded connections. This property was the reason for assigning it a common name - "liquid key".
Processing wet metal parts with it allows you to remove moisture from the surface and create a protective layer on it.
Transformer "Tsinkar"
Contains manganese and zinc salts. Available in spray bottles. But according to user reviews, it is safer to apply it to the surface with a brush. When processing painted parts, it eliminates corrosion without damaging the adjacent paint layer.
NEOMID anti-rust for metal
The main application is cleaning corrosive areas on car bodies. Copes with fresh and old dirt. To remove thick layers, the affected area is treated several times.
Sanox cleaning agent
The basis is made by compounds of citric and oxalic acids. It is used to eliminate dirt and stains on ceramics. But Sanox is also used against rust on metal. To do this, it is enough to wipe the affected area with a swab dipped in a cleaner.After completely removing traces of corrosion, rinse with water and dry with a cloth or hairdryer.
Folk ways
The main rules when using any of these products are strict adherence to the exposure time to prevent damage to the product itself and thorough drying to delay re-corrosion.
Aluminium foil
In this case, the foil acts as an analogue of an abrasive brush, that is, it has an exclusively mechanical effect. A piece of foil must be crumpled into a tight ball that can be used to clean the surface. It should be borne in mind that this method is suitable for light pollution on household appliances. But for cleaning rusty pipes, it is better to use chemicals.
Use a crumpled ball of aluminum foil to remove rust from these areas and give them a sleek look.
Vinegar
It is worth using white vinegar, as its flavored and colored counterparts will not only not help remove dirt, but can also leave new ones. The procedure for this is as follows:
- pour vinegar into a spray bottle;
- apply the substance to the site of the lesion;
- leave the treated surface for a couple of hours;
- clean off plaque with a metal brush;
- wash off traces of vinegar with warm water;
- dry the treatment area in the sun or wipe thoroughly with a rag.
Vinegar is the most popular and affordable way to remove corrosion deposits from metal products and more.
Soda
Effective enough for small soiling. Soda must be diluted with water to obtain a pasty mass, which should be applied to the surface in a thick layer. After half an hour, the plaque is easily cleaned off with a metal sponge or brush.
The procedure can be repeated if necessary.
Lemon acid
A fairly effective tool that has a number of advantages:
- availability;
- preservation of the appearance of the paint covering the metal product;
- absence of aggressive chemicals in the composition;
- harmless to the skin of the hands (the use of gloves is still recommended).
This method removes corrosion in all hard-to-reach areas.
Before starting work, the metal surface must be degreased, which can be done with a dishwashing detergent. Then the product is placed in a concentrated solution (80 g of citric acid per 100 ml of warm water) for several hours. In this case, the reaction begins within a few minutes, which is signaled by the formed bubbles. After the completion of the reaction, the surface must be rinsed under running water, if necessary, removing the remaining contamination with a metal brush.
Oxalic acid
The algorithm for working with this substance is as follows:
- wash the rusted product using any dishwashing detergent;
- dry thoroughly;
- mix six teaspoons of oxalic acid with 300 ml of water;
- immerse the object in the solution for half an hour;
- remove rust residues with a stiff brush;
- dry the product thoroughly.
When using it, you must adhere to safety precautions: wear safety glasses and rubber gloves.
Hydrochloric acid
A 2% hydrochloric acid solution will effectively clean the white thing from rust. In this case, the object to be cleaned is simply enough to treat it with acid until the contamination disappears. After that, it is necessary to rinse it in a solution of ammonia and water (a couple of tablespoons of ammonia per liter of water).
With this tool, you can effectively remove rust from white things.
Hydrogen peroxide
This substance has oxidizing and reducing properties, which allows it to effectively remove traces of rust from objects such as bathtubs, toilet bowls, knives or tools.
Hydrogen peroxide is used according to the following scheme:
dilute four tablespoons of trisodium phosphate powder in three liters of water;
carefully pour 50 ml of peroxide into the solution, dividing into five portions;
soak the product in the mixture for half an hour;
thoroughly wipe the area to be treated with a regular sponge, then leave the product for another ten minutes;
rinse the surface with clean water.
Coca-Cola
This drink contains phosphoric acid, and therefore can be used to remove rust. It is enough to place a damaged product in a liquid for 25-30 hours, after which it should be rinsed with clean water and wiped dry.
Surely many housewives have heard that you can remove rust with cola.
Why does rust appear
Metals are subject to rust due to the fact that they are mined and used not pure, but in the form of chemical compounds with oxygen, carbon, water, sulfur and other elements. For pure metals, corrosion is not terrible, since they do not tend to form compounds with environmental substances. However, there are few such materials - silver, gold and platinum.
There are a huge number of different ways to remove rust from metal, but not all of them work as we would like.
Most of the contaminated metals mined are smelted, refined and recovered to make them pure. However, in this case, they become unstable and tend to react with elements of the environment. So, when a metal comes into contact with air, an oxide is formed, and with moisture, a hydroxide is formed. These processes are natural for iron and are called corrosion, and their result is rust.
Even if it is not possible to remove the rust the first time, then with some effort, you can always return the object to its original appearance.
Since metals are mainly used as durable materials, which are the basis for various structures, they are constantly in contact with the environment and the corrosion process is inevitable. If air oxygen and moisture are constantly present, then after a while the iron can be destroyed to the ground, completely turned into rust.
There are various ways to remove corrosive deposits if the process has not gone too far.
However, corrosion is not a very fast process, so iron products are destroyed for quite a long time. In addition, new methods are being invented to delay the appearance of rust as much as possible.
However, rusty deposits can often be found on various non-metallic surfaces such as tiles or bathtubs. This is due to rusting plumbing fixtures and other equipment with metal elements that are exposed to the environment. Rust is then carried away by water, contaminating surfaces in addition to limescale. However, all such coatings are fairly easy to clean.
Household chemicals are the most effective means of removing rust.
It is worth removing rust in the following cases:
- Before gluing or painting metal products. Traces of corrosion will prevent glue or paint from firmly bonding to the metal surface, and therefore must be removed.
- For protection against destruction. In order for a metal product to serve for a long time, it must be promptly cleaned of iron oxides and covered with anti-corrosion materials such as zinc oxide.
- For aesthetic reasons.
Folk methods
 Potatoes from rust in a cast iron pan
Potatoes from rust in a cast iron pan
The folk methods of rust treatment used by our grandparents are still relevant to this day.
- Formalin is a popular anti-corrosion method. A solution with this component will help remove rust from surfaces. It must be diluted in equal proportions with water, add soda and ammonia. Degreased products that require cleaning must be dipped in the solution for half an hour.
- Hydrogen peroxide. You can get rid of rust with hydrogen peroxide. It is enough to pour the contents of the vial onto a metal surface.After a while, clean and dry.
- Potato. One medium potato and table salt can help remove rusty pots and cast iron pans. The potatoes are cut in half and salted. Then rub the rusty surface with half of the tuber.
- Lactic acid.
- Ketchup or tomato. To remove iron oxide, use ketchup or tomato paste. You just need to spread the tomato on the right place and wait.
- Salt and baking soda are great for removing impurities.
How to prevent rust
It is unrealistic to protect open metal from contact with air and moisture, therefore, its surface must be covered with protective layers. It can be any paint applied to a previously primed surface.
Along with the rust remover, you can purchase special sealants, that is, compounds that reliably block the access of moisture and oxygen to the metal protected by them.
WD-40 products include a silicone based lubricant that is used to coat parts to create an impermeable layer after rust removal.
It is recommended to protect threaded connections by dipping them in graphite oil before screwing them in.
Parts and tools intended for long-term storage are covered with a thick layer of petroleum jelly or grease.