Content
Crystals appear when a substance passes from any state of aggregation to a solid state. The main condition for the formation of crystals is a decrease in temperature to a certain level, below which particles (atoms, ions), having lost their excess thermal motion, exhibit their inherent chemical properties and are grouped into a spatial lattice.
Methods and factors of crystal nucleation
At temperatures measured in thousands of degrees, none of the substances known in nature can exist in a crystalline state. The second important condition is pressure. Temperature and pressure are the thermodynamic conditions for the existence of a crystalline substance. A highly heated substance, when cooled, can go through the stages of a gaseous mixture, liquid, melt, solid state. Therefore, three ways of crystal formation are possible.
- Crystallization by sublimation - the transition directly from the gaseous state to the solid. In this case, crystals are formed directly from the vapor, bypassing the liquid phase. An example is the sublimation and recrystallization of iodine. In nature, this process occurs in craters, volcanic cracks (deposits of ammonia, sulfur, etc.). In winter, with clear frosty weather, snowflakes form in the air.
- Solid state crystallization - transition from solid to solid state. Two processes are possible here. First - a crystalline substance can be formed from an amorphous one. Thus, glass and glass-containing volcanic rocks crystallize over time. Second process - recrystallization: the structure of some substances is destroyed and new crystals with a different structure are formed. Recrystallization phenomena are widespread in nature and lead to the formation of new minerals, rocks and ores. All metamorphic rocks are recrystallized to one degree or another. Under the influence of temperature, pressure and other factors, limestone, for example, transforms into marble, clay rocks - into phyllites and crystalline schists, quartz sandstones - into quartzites.
- Crystallization from melts and solutions - the main method of crystal formation in nature. This is how massive crystalline rocks - granites - are formed from fiery liquid silicate melt (magma). Salt crystals are deposited at the bottom of lakes, bays and in the sea. Artificial crystals are grown from melts and solutions (for example, technical and precious stones: piezoquartz, carborundum, ruby, diamond, sapphire, etc.).
Thus, the main condition for nucleation is hypothermia or oversaturation. The nucleation of crystals is hypothermia or oversaturation. The nucleation of crystals can proceed on its own. But sometimes for the growth of crystals, the presence of the smallest crystals of the crystallized substance itself or particles of other solid substances close to it in structure is sufficient. The process of crystal formation is abrupt, with the release of energy, with a rearrangement of particles, with a sharp change in the initial properties. The crystallization ability of different substances is not the same, it is determined by the number of crystallization centers formed per unit time per unit volume, and the rate of crystal growth.At a high rate of formation of crystallization centers, many small crystals appear; at a small number of centers, large crystals appear.
How to grow salt crystal at home
You can independently conduct experiments on growing crystals. A weighed portion of one or another salt (alum, copper sulfate, etc.) is preliminarily prepared. Pour the weighed portion into a chemically glass or porcelain beaker and pour the required amount of water using a graduated beaker. Covering the glass with a round (watch) glass, heat its contents to accelerate the dissolution of salt in water. Then filter the resulting solution.
Table 1 - Solubility of salts (in grams) in 100 cm3 of water.
|
Temperature, ⁰С |
Potassium aluminum alum KAl 12H2O | Sodium nitrate
NaNO3 |
Magnesium sulfate
MgSO4 7H2O |
Copper sulfate
CuSO4 5H2O |
| 0 | 3,9 | 73 | 76,9 | 31,6 |
| 10 | 9,5 | 80,6 | 93,8 | 37 |
| 20 | 15,1 | 88,5 | 115,9 | 42,3 |
| 30 | 22 | 96,6 | 146,3 | 48,8 |
| 40 | 30,9 | 104,9 | 179,3 | 56,9 |
Place the filtered liquid in a special glass with a wide bottom and low walls. In the glass, the solution cools and evaporates intensively, which is facilitated by the characteristic shape of the glass, which creates a large evaporation surface. As a result of cooling and evaporation, first a saturated and then a supersaturated solution is obtained (it contains an excess of a solute). At the same time, crystals begin to fall out in the crystallizer. The next day (after preparing the solution), you need to select a few or one of the dropped crystals, carefully drain the solution into a clean crystallizer and place the selected crystals - "breakfasts" there. "Breakfasts" are particles that can cause crystallization. To obtain a well-cut (isometric) crystal, it should be grown on a hair or silk thread. Crystals grown at the bottom of the vessel, being constrained in growth, will acquire an irregular shape (flattened, elongated). After a certain period of time, when the solution becomes small, a fresh solution should be prepared and the crystal should be transferred into it. List of equipment for growing crystals: reagents, mortar (porcelain), scales with weights (pharmaceutical), two glasses (chemical or porcelain), beaker, burner, asbestos mesh, round watch glass, glass rod for placing the solution, funnel, filter paper, funnel stand, glass with a wide bottom, tweezers, thermometer. A convenient material for producing well-formed crystals is alum. The solubility of alum in hot water is much greater than in cold water, so we can speed up the process by cooling the saturated solution. Dissolve alum in hot water to the limits of solubility; you get a saturated solution. Experience shows that 25 g of alum can be dissolved in 200 g of hot water. Crystals of the regular shape characteristic of alum - octahedrons, grow on silk threads dipped in a solution. Remove small and irregular crystals from the thread and leave one better one, which will gradually build up. The alum substance evenly settles on the edges of the free-growing crystal. When a saturated solution (75 g of chromium alum per 20 g of water) is cooled to a temperature of 11 ° C, crusts of fine-grained crystals fall out on the bottom of the vessel. Regular crystals of chromium alum in the form of violet octahedra grow on threads dipped in solution. Crystals of aluminum alum can grow in a solution of chromium and vice versa, since both have the same type of spatial lattice. Place a purple chrome alum crystal growing on a string in a saturated solution of aluminum alum - you get a two-layer crystal with a purple inner octahedron and a colorless outer.
The most successful result is obtained when growing crystals of copper sulfate from a solution with a concentration of 29.2% when it is cooled to 13.5 ° C. Dissolve 82.5 g of powdered copper sulfate in 200 g of water while heating. Pass the solution through a paper filter. After 14-15 hours, well-formed crystals up to 1.5 cm in length will precipitate.In addition to alum and copper sulfate, potassium dichromate crystallizes well. Self-faceting experiment: shape the grown alum crystal (filed) into a ball shape, dip it back into the saturated mother liquor and watch it grow. After 1-2 days, you will notice that faces appear on the ball, and after a week, instead of the ball, a regular octahedron is formed again.
Growing crystals is a very interesting hobby, especially since crystals are very beautiful, and maybe they can be used in magic as well. The article below is written by me, but uses the knowledge gained in books on growing crystals - that is, this article is a retelling of them and my own experience (during my school years it was my anniversary to study it, maybe I will return to this).
The substance has several states of aggregation, among which we all know - solid, liquid and gaseous. Crystal is
solid state of matter. It is characterized by the fact that the molecules in it are arranged in a certain way.
Crystals are a very beautiful, mesmerizing natural phenomenon - I think many will agree with this.
In nature, there are beautiful gems or non-precious stones that have the correct shape.
People have learned to grow artificial gems. This requires powerful hardware. But some crystals can be grown right at home, without any equipment. These will, of course, not be artificial diamonds and rubies, but crystals of salt or copper sulfate are also very beautiful.
Below I will provide several ways to grow them.
Crystal formation. Solubility of the substance.Here and below, we will talk about those crystals that consist of substances soluble in water and will be grown in water.
The formation of a crystal is a gradual "sticking" of molecules of a substance to a small crystal or to something else -
seed
... So during this adhesion, the crystal grows. The task of the crystal growers is to make this substance stick. The easiest way is to use solutions.
As you know, a certain amount of a substance dissolves in water. If, say, you dissolve salt in water, then gradually adding more and more salt, you will see that it does not dissolve anymore. Such a solution (in which the substances are dissolved to the limit) is called concentrated. To grow a crystal, you need a concentrated solution, and then you need to gradually remove the maximum of the substance that can be dissolved in water. Then the excess substance will have nowhere to go and it will settle on the seed.
There are two ways to do this. The first is to remove water, but not the substance itself - that is, evaporation. The water evaporates, but the substance remains. Thus, there is less water, and the same amount of substance. And since the solution is concentrated, its amount begins to exceed the maximum that water can contain and part of the substance settles.
The second way is to simply reduce the solubility of the substance (that is, the amount of the substance that can be dissolved in the volume of water).
The solubility of a substance is not a constant, it depends on the temperature of the water. The hotter the water, the greater the solubility of the solid. Therefore, by making a concentrated solution in hot water, and then cooling the water - you will get the same effect - a significant part of the substance will cease to "fit" into the water and settle.
Now it becomes clear how to grow crystals - just take a concentrated solution of a substance, pour it into a jar, put a seed there and either cool the water or evaporate (you can do both).
However, there are a few more important points.
The rate of formation of crystals - if you evaporate the water too quickly, then the crystal will not have time to grow, and you will have a lot of small crystals or even crystalline "moss". The substance needs time to "knead" in the form of a crystal.
And the second is the seed. I already wrote about it above.In general, matter tends to settle and crystallize on some irregularities
or a similar substance. If you take a smooth jar and set it to grow unseeded, many small crystals will grow.
But if you want to grow a solid crystal, you should make sure that there are fewer items in the jar. For example, if you are growing it on a thread, then it is better to use a smooth thread or wire, rather than fluffy thread, otherwise it will most likely overgrow
many crystals. Although it is good to use a fluffy thread to grow a crystal "necklace".
Also one of the interesting properties of a crystal is its shape. There are several basic crystal shapes. So, for example, cubic crystals will always grow from table salt. Of course, this does not mean that a perfect cube will grow out of it. Usually, nevertheless, crystals
are perfect. But its form will be based on a cube. That is, irregularities and "extra" crystals will be cubic. And it will not be rhomboid in any way, for example.
One more thing, quite obvious, but I'll write it anyway. The substance from which the crystal is grown must be homogeneous and crystalline. That is, a crystal can be grown from salt, but sea salt will not work for this (since it is heterogeneous), just like many organic soluble substances.
The first crystal. Well, I have described a certain theory. Now I will describe a little practice. There are polycrystals and monocrystals. Polycrystals are a group of many small crystals. So crystalline "obesity" is a polycrystal. A single crystal is one large crystal. If we consider table salt, then this is, in fact, a lot of small single crystals. And if many crystals grow together, forming a kind of "hedgehog" or something similar, it will be a polycrystal.
I will describe the technique of growing a single crystal - it is more difficult. But knowing the principle of growing a single crystal, you will be able to grow polycrystals. Of course, it is difficult to achieve an ideal single crystal, but you can achieve something close to this. So, I will describe the instruction as
grow a large "gem". The task is to grow as much as possible and as correctly as possible.
It is often advised to start with table salt. But it is quite difficult, in fact, and for a very long time. Since the fastest way to grow crystals is by cooling, and the solubility of table salt is very weakly dependent on temperature. Almost as much salt can dissolve in boiling water as in almost ice water. And the evaporation method is very long.
Therefore, I suggest starting with copper sulfate - you can buy it at the hardware store. It's such a blue substance. Watch out, it's poisonous! Therefore, handle it carefully, try not to get it on your skin (especially on scratches) and do not sprinkle it at all.
It produces very beautiful blue crystals. And in vitriol, the solubility significantly changes depending on the temperature, therefore it is very convenient for growing crystals from it.
The principle of cultivation is as follows.
1. Prepare your seed (a small crystal, or a large one that you want to enlarge). I will describe where to get it below.
Hang it in a jar where your crystal will grow. It is important to hang it up so that it can grow comprehensively. To hang - take a thread or wire and wrap a crystal with it on one side, and a pencil or stick on the other, which will be placed on
the top of the jar (so that the crystal hangs down and is just below the center of the jar). Take the wire with a margin - since the crystal will increase, therefore, the dimensions of the can, and therefore the height, may also increase. That is, wrap a few circles around the pencil. Take a thread or wire so that it is as smooth as possible. Of course, if there is none at all, then this is not critical (in the last paragraph I will describe why).
2. Take warm water (but not boiling water, it will cool too quickly). Make a concentrated solution.To do this, pour vitriol into a jar of water and stir. Add cupros until it stops dissolving. Then drain the water into the jar where your crystal is suspended (so that there are no extra crystals). Yes, the dishes should be clean - the cleaner the better. Otherwise, the dirt will form a lot of seeds, and dissolving in water, it will reduce the integrity of the substance. Of course, styling is difficult to achieve, but you should not take dirty dishes.
3.Place the jar in a warm place. Warm so that the water does not cool too quickly. You can even cover with something. It all depends on your starting temperature. If it is not too high, then you can just in the room. Wait until the water cools down to the temperature of the place where you put the jar. Then move the jar to a cooler place, such as a window. And so on until the temperature becomes minimum (although I do not recommend putting vitriol in the refrigerator, so the minimum is the window temperature). In general, cool while you can. The main thing is not to put boiling water on the window right away.
4. When the solution has cooled, pour it into another jar. What will you do with him, I do not know. You can evaporate so that the good (that is, the vitriol remaining in it) does not disappear, you can pour it out (if not sorry), you can warm it up and dissolve a new vitriol in it and repeat the procedure. The main thing that you need is to prepare the crystal for the next session. Usually, on it (as well as on a thread), in spite of everything, extra crystals grow. This is inevitable - especially in this way (it's still not the most accurate way). But this is not scary - you just need to carefully clean out the excess crystals. Do this carefully, try not to damage the main one. Then repeat the previous points.
This method requires your participation, as the growth rate is amazing. Usually they talk about several days, but here the visible results can be less than an hour! At this rate, you can grow huge crystals. I heard that the fans raised the kind that a few people could lift them! Therefore, every half hour you should rearrange the can, and then change the solution.
Of course, you can hardly be on duty around the clock, so while you are busy, you can simply leave the jar in place - although the temperature will not change, the water evaporates. Here the saying goes: "The soldier is asleep, the service is going on."
You can grow polycrystals, too, which is also quite a beautiful thing. If you put a woolen thread in a warm solution and hang it in an arc, you will get an excellent necklace (although I do not recommend wearing it as an adornment).
Seeding.You will need a seed before you can breed the main crystal. Let's take a small crystal as it. Of course, you can take small crystals that are in the jar with vitriol. But they are too small. Therefore, you can simply pour it into a small, clean container, like a saucer or something similar to a warm concentrated solution of vitriol. About half an hour later, many small crystals will appear at the bottom. Choose the largest and correct one.
At first, I advise using small containers for crystal growth (the smallest jar that is), and taking the base water temperature not too high, a little more than room temperature. The fact is that if you immediately start growing a small crystal at the same rate as a large one, then extra crystals of a size similar or even larger than the seed itself may grow there. So, until the crystal gets stronger, it is worth showing a little more patience.
Storage of crystals.Well then, here you are and have grown your crystal. Big, correct, beautiful! I think you will not be very pleasantly surprised when you find that it is covered with a blue bryophyte crust, lying calmly on your shelf. There are many reasons for this - corrosion, moisture, and so on. The crystal must be protected. Use a protective varnish, coat it thoroughly and all over.You can also store it in closed, protected places, although this is not the best solution if you want to have it on the shelf. And besides, varnishing will protect against poisonous vitriol. This does not mean that after you touched it, you should not wash your hands, but still the danger will be much less. Remember also that vitriol crystals are fragile. Do not beat or crush them, otherwise you will lose your miracle.
Copper crystals!In fact, it is not only crystals of soluble substances that can be grown. You can grow a real copper crystal! No, you don't need a laboratory and powerful equipment! It's easy to do at home. You just need to know the chemistry just a little bit, and that's it. And as a result, you get a real copper crystal. Yes, metals are in principle crystalline, but there will be a solid metal crystal.
How is such a miracle possible?
It's simple. Consider what salt is. Salt is a combination of a metal with an acid. Cooking ol (the one we eat) is the result of the reaction of sodium and hydrochloric acid. Copper sulfate is the result of the reaction of sulfuric acid and copper. That is, salt can be said to have two components - metallic and acidic.
Salts have a remarkable property - they tend to have the "strongest" metallic component. Therefore, if the salt reacts with a metal that has a greater activity than the metal component of the salt, then it will push its metal out of itself and take the more active one. And to get such a reaction, it is enough to dissolve the salt in water and put an active metal in this water.
You probably haven't seen this reaction with table salt, simply because its metal component, sodium, is extremely reactive. Few metals are more active than sodium (such as potassium). And metals of similar activity react with whatever they can - if you throw potassium into water, it will react faster with it - that's why they practically do not occur in pure form.
But copper is a completely passive metal (even more passive - noble, like gold).
The secret is to put a more active metal - for example, iron - into the solution of copper sulfate. Then copper will begin to be pushed out and crystallized, and geoezo will become the metal component of the salt - and you will get iron vitriol. Also, aluminum is more active - it is generally terribly active, but due to its protective shell (oxidation) it almost does not react with anything. But if you use a catalyst, it will start to react. Table salt will be used as a catalyst - if you take a solution of table salt and copper sulfate and throw pieces of aluminum there, then the water will almost boil - such a violent reaction will be.
This is good for chemical experiments, but bad for the task of growing crystals - because instead of crystals, "tina" will be formed - that is, myriads of completely microscopic crystals. It takes an extremely long time to grow metal crystals. In general, I did it only once, and then the crystal was about a millimeter.
In order for copper to have enough time to crystallize, it is necessary to slow down the process as much as possible. The crystal will grow for months.
I am writing the technique itself, and I will provide a theoretical basis for it.
First, grow a few copper sulfate crystals (small, like a seed). This is necessary because crystals dissolve less readily than powder. Large ones are optional.
Then take a long jar - its length is important. Put the crystals down, pour table salt almost to the top. At the very top, place an active metal - iron or aluminum. Fill with cold water and forget about the copper crystal for several months. I would recommend placing a lot of such crystals at once - this way the probability of success is greater.
You will see how the solution gradually goes up. It will be green, since a solution of copper sulfate mixed with a solution of sodium chloride is green. He will gradually get to the top of the can and the reaction will begin.Then, from a beautiful green, the water is painted in a dirty rusty color - it means that the reaction has already started. It will be ready when there are practically no crystals at the bottom (you will see it through the glass). When placing a copper crystal, you can experiment with cans of different heights and with other parameters (including metal).
Iron is generally better suited for the growth of copper crystals, but aluminum is also possible (here salt will be both a catalyst and a moderator).
As you can imagine, you can grow crystals of other metals, for example, iron from ferrous sulfate.
When you take out the crystal, carefully pour and pour the contents of the jar into a bowl. The crystal may not be at the very top (where, in theory, it might be) - it may get lost in salt - on the road or at the very bottom. Plus, it will most likely be small, so search everything.
Later I will give tables of solubility (depending on temperature) and metal activity.
From SurWiki
Kuvatova Nasima
Research work: File: Crystals.rar
Presentation: File: Crystals.ppt
Source (s): Growing crystals and their application
Goals: find out and show that a crystal, no matter how it is obtained, obeys the law of symmetry. Determine the main areas of application of crystals.
Tasks: Acquisition by students:
- general educational skills: work with scientific literature, conduct observations, exercise self-control and introspection.
- special knowledge and skills on this project topic, the ability to navigate in the information space, to independently design their knowledge.
- research knowledge and skills: formulate hypotheses, highlight problems, plan an experiment in accordance with a hypothesis, draw conclusions.
Equipment and reagents: scales, chemical glassware (cups, funnels, flasks), stands, wire, filters, water, salts (potassium alum, nickel sulfate, potassium dichromate, copper sulfate, aluminum nitrate).
The generation of us, choked with number X, who measured the clock on the universal scales of the universe ... A generation of those who know the dimensionality of crazy pages And do not believe in dogmas, anathemas and predictions ... Storms of letters and numbers, visions and dreams are flying in. - the basics of BASICS Crumbled with fragments of glare, jubilant ink But, like a wolf, flair, we are each other ... "by sound" and "in a syllable" ... As "by smell - flying in" ... and ... the wounded fellow tribesman is ready ... And in the morning: "Bye! Come! .. These are the Gods, this is the threshold ... - Don't forget about the CRYSTAL! .. - About the LIBRA ... - I'm waiting for the CUT ... "/ D. Bloshchinsky /
Updating

Crystal, as a mysterious and beautiful part of nature, has attracted people's attention since ancient times.
The crystal usually serves as a symbol of inanimate nature. However, the line between living and non-living is very difficult to establish, and the concept of "crystal" and "life" are not mutually exclusive.
Natural crystals have always piqued people's curiosity. Their color, brilliance and shape affected the human sense of beauty, and people decorated themselves and their homes with them. Superstition has long been associated with crystals; as amulets, they were supposed not only to protect their owners from evil spirits, but also to endow them with supernatural powers.
Later, when the same minerals began to be cut and polished like precious stones, many superstitions were preserved in the talismans "for good luck" and "their stones" corresponding to the month of birth. All natural precious stones, except opal, are crystalline, and many of them, such as diamond, ruby, sapphire and emerald, come across as beautifully cut crystals.
The most famous examples of crystals are ice, diamond, quartz, rock salt.Most solids do not have the regular geometric shape of a polyhedron with flat faces and sharp edges characteristic of crystals. The word "crystal" comes from the Greek - "ice".
Water is a "universal" solvent

Water is the most common solvent for solid, liquid and gaseous substances. It is well known from everyday life that if some substances dissolve in water, then solutions are formed.
Homogeneous homogeneous systems containing two or more substances are called solutions. Solutions can be not only liquid, but also solid, for example, glass, an alloy of silver and gold. Gaseous solutions such as air are also known. The most important and common are aqueous solutions.
According to modern concepts, dissolution is the result of the chemical interaction of a solvent and a solute, with the formation of molecular compounds. In aqueous solutions, these compounds are called hydrates, and in non-aqueous solutions, they are called solvates.
A saturated solution is a solution that is in equilibrium with an excess of a solute. It contains the maximum possible amount of solute. The concept of "saturated solutions" should be distinguished from the concept of "concentrated solutions". A concentrated solution is a solution with a high solute content. If the concentration of the solution does not reach the saturation concentration under these conditions, then the solution is called unsaturated. By carefully cooling a hot saturated solution (for example, copper sulfate or Glauber's salt), so-called supersaturated solutions can be obtained.
Crystals in nature
Ice and snow crystals

Crystals of frozen water, i.e. ice and snow are known to everyone. These crystals cover the vast expanses of the Earth for almost six months (and in the polar regions all year round), lie on the tops of mountains and slide off them with glaciers, float as icebergs in the oceans.
The ice sheet of a river, a massif of a glacier or an iceberg is, of course, not one big crystal. The dense mass of ice is usually polycrystalline, i.e. consists of many individual crystals. You cannot always distinguish them, because they are small and all have grown together. Sometimes these crystals can be discerned in melting ice, for example, in the ice floes of the spring ice drift on the river. Then it can be seen that the ice consists, as it were, of "pencils" fused together, as in a folded pack of pencils: the hexagonal columns are parallel to each other and stand upright to the surface of the water; these "pencils" are ice crystals.
It is known how dangerous spring or autumn frosts are for plants. The temperature of the soil and air drops below zero, the subsoil water and plant juices freeze, forming needles of ice crystals. These sharp needles tear the delicate tissues of plants, the leaves shrivel, turn black, the stems and roots are destroyed. After frosty nights, in the morning in the forest and in the field, one can often observe how "ice grass" grows on the ground. Each stalk of this herb is a transparent hexagonal crystal of ice. Ice needles reach a length of 1-2 cm, and sometimes reach 10-12 cm. It happens that the ground is covered with plates of ice standing upright. Growing out of the ground, these ice crystals raise sand, pebbles, pebbles weighing up to 50-100g on their heads. Ice floes are even pushed out of the ground and carried up by small plants. Sometimes an ice crust envelops the plant and the root shines through the ice. It also happens that a brush of ice needles together raises a heavy stone, which cannot be moved by a single crystal. The crystal "ice grass" sparkles and burns with an iridescent sheen, but as soon as the rays of the sun warm up, the crystals bend towards the sun, fall and quickly melt.
On a frosty spring or autumn morning, when the sun has not yet had time to destroy the traces of night frosts, trees and bushes are covered with hoarfrost.Drops of ice hung on the branches. Look closely: bundles of thin six-sided needles - ice crystals - are visible inside the ice drops. The leaves covered with frost look like brushes: like bristles, shiny hexagonal columns of ice crystals stand on them. The forest is decorated with a fabulous wealth of crystals, crystal dress.
Every single ice crystal, every snowflake is fragile and small. On snowflakes, it is easiest to make sure that the shape of the crystals is correct and symmetrical. The shapes of snowflake stars are surprisingly diverse, but their symmetry is always the same: only six rays. Why? This is the symmetry of the atomic structure of snow crystals. This does not only apply to snow. The forms of crystals can be very diverse, but the symmetry of these forms for each substance is the same, it is determined by the symmetry and regularity of the atomic structure of a given substance. A snowflake can only be six-rayed - this is the symmetry of the structure of snow crystals.

Crystals in the clouds
Ice crystals, the bizarre patterns of which we admire in snowflakes, can destroy an airplane in a few minutes. Icing - a terrible enemy of airplanes - is also the result of crystal growth.
Here we are dealing with the growth of crystals from supercooled vapors. In the upper atmosphere, water vapor or water droplets can persist for a long time in a supercooled state. Hypothermia in the clouds reaches -30˚C. But as soon as a flying plane bursts into these supercooled clouds, violent crystallization immediately begins. Instantly, the plane is covered in a pile of rapidly growing ice crystals.
Crystals in caves
All natural waters - in oceans, seas, lakes, streams and underground springs - are natural solutions, they all dissolve the rocks they meet, and complex crystallization phenomena occur in all these solutions.
Crystallization of underground waters in caves is especially interesting. Drop by drop water seeps in and falls down from the vaults of the cave. At the same time, each droplet partially evaporates and the substance that was dissolved in it remains on the ceiling of the cave. This is how a small tubercle gradually forms on the ceiling of the cave, which then grows into an icicle. These icicles are made of crystals. One drop after another falls steadily day after day, year after year, century after century. The sound of their fall is muffled under the arches. Icicles all stretch and stretch, and towards them the same long columns of icicles from the bottom of the cave begin to grow upwards. Sometimes icicles growing from above (stalactites) and from below (stalagmites) meet, grow together and form columns. This is how patterned, twisted garlands and bizarre colonnades appear in underground caves. The underground halls are fabulously, unusually beautiful, decorated with fantastic piles of stalactites and stalagmites, divided into arches by stalactite gratings. In nature, crystals of irregular shape are found incomparably more often than regular polyhedrons. In river beds, due to the friction of crystals against sand and stones, the corners of the crystals are erased, multifaceted crystals turn into rounded pebbles; from the action of water, wind, frost, crystals crack, crumble; in rocks, crystal grains prevent each other from growing and acquiring irregular shapes.
Photographs of natural crystals in foodstuffs.
Azishskaya in the Krasnodar Territory (Republic of Adygea).
Crystals growing from below

Crystals growing from above

Column hall grown from crystals

Methods for growing crystals from solutions
Crystallization by means of "seeds"
The phenomenon of salt crystallization is not difficult to reproduce experimentally. Dissolve a pinch of plain table salt in the water and pour the salt water onto a saucer. When the water evaporates, look through a magnifying glass, and you will see that the correct white cubes of crystals with stripes of edges remain on the saucer. Crystals of rock (table) salt formed from the solution before your eyes.So in miniature, you can observe the phenomenon of crystallization of a solution, which in nature, in salt lakes and in subsoil waters, occurs on a gigantic scale.
Why do crystals stand out from the solution? To understand this, one should get acquainted with some of the properties of solutions.
Try to dissolve table salt in water: 70 grams of salt will dissolve in a faceted glass of water, and if you pour salt further, it will stop dissolving and will settle to the bottom. You will see the same thing with sugar: about twenty teaspoons of granulated sugar will dissolve in a glass of cold water, and then the sugar will also settle to the bottom without dissolving. Only a very specific amount of sugar (194 grams), table salt (35 grams) or any other substance can dissolve in 100 grams of cold water. The amount of a substance that can dissolve in 100 grams of water is called the solubility of this substance in water; for example, the solubility of sodium chloride in water at room temperature is 35 grams. Solubility is temperature dependent. Try to dissolve sugar not in cold water, but in hot water, and you will see that as the temperature rises, the sugar solubility increases. For different substances, solubility depends on temperature in different ways.
So, at any given temperature, only a strictly limited amount of a substance, determined by its solubility, can dissolve in water.
Take a glass of hot water and add any crystalline substance that is soluble in water: hyposulfite, soda, boric acid, alum. If you get large crystals, crush them into powder first. Pour as much powder into a glass of hot water as it can dissolve. When the powder completely stops dissolving and begins to settle to the bottom, pour the resulting solution into another glass so that not a single grain of powder falls on the bottom of the glass with the solution. To do this, filter the solution through filter paper or a clean cloth. In the resulting solution, the amount of the substance just corresponds to its solubility at a given temperature; the solution is “saturated”, and it can no longer absorb a single grain of the substance. This solution is called saturated. Now leave the glass with the solution and let it cool. With cooling, the solubility of almost all substances decreases; while our solution was hot, in a glass of water, say, 12 tablespoons of the substance were dissolved, whereas at room temperature only 10 tablespoons of this substance could dissolve in it. Thus, now there will be an excess substance in the solution. In other words, at a high temperature the solution was saturated, and when it cooled down, it became oversaturated. Such a supersaturated solution cannot exist for a long time, so the excess substance is released from the solution and settles to the bottom of the glass. Examine through a magnifying glass and you will see that this precipitate is composed of crystals.
A dissolved substance crystallizes from supersaturated solutions because there is too much of it in the solution - more than the solution can hold in itself.
Transparent crystals of potassium alum grew out of the aqueous solution within a few hours. To prepare an aqueous solution of potassium alum, it is necessary to dissolve 48 g of potassium alum, ground into powder, in 400 cm3 of hot water. If you dissolve 60 g of alum, you get a solution oversaturated at 15˚C by 12 g. Therefore, it is necessary to take hot water: more than 48g would not dissolve in cold water. A supersaturated solution will begin to crystallize if any "seed" gets into it. To do this, it is enough to slightly open the lid of the jar for one or two seconds: alum dust particles from the air will get into the solution. You can also add a few grains of alum to the solution with a needle.Once in a supersaturated solution, alum dust particles in it will immediately begin to grow, and if crystallization has begun in the solution, it will not stop until the entire excess of the solute is released.
You can also grow one large crystal. To do this, a small crystal, a "seed", must be placed in the uncooled solution or brought on a thread. At first it will dissolve a little, and then it will begin to grow.

If an object containing a lot of seeds is placed in a vessel with a solution, it will be overgrown with crystals. Dip a thread containing crystalline dust particles into the solution - crystals will begin to settle on them, and as a result, a "string of beads" of multifaceted crystals grows. Such threads can compete in beauty with artificially cut beads, but, unfortunately, crystals grown from aqueous solutions usually fade very quickly and easily break down. This is the difficulty in using them in technology.
You can make figurines from crystals.

To do this, you need to prepare a frame of wire wrapped with ordinary threads or cotton wool, dip it in a saturated solution, immediately remove it and dry it at room temperature. The threads are impregnated with the solution and when dry, tiny crystals are formed on them, which will later serve as "seeds". And then lower this framework into the solution and grow crystals on it. If you put a collapsible synthetic Christmas tree into the solution, having previously wrapped its trunk and branches with threads, you can grow a "snow-covered" Christmas tree. To do this, it is better to take not alum, but potassium dihydrogen phosphate (KH2PO4) or ammonium dihydrogen phosphate (NH4H2PO4) - wonderful crystals that grow for devices that control the Lazarus beam. Their solubility per 100 g of water:
| At a temperature | 20˚C | 40˚C |
| KH2PO4 | 22.5g | 33g |
| NH4H2PO4 | 36.5g | 56.6g |
Main applications of crystals
Living on an Earth composed of crystalline rocks, we certainly cannot get away from the problem of crystallinity: we walk on crystals, build from crystals, process crystals in factories, grow them in laboratories, widely use them in technology and science, eat crystals, heal them ... The study of the variety of crystals is engaged in the science of crystallography. She comprehensively examines crystalline substances, examines their properties and structure. In ancient times, crystals were believed to be rare. Indeed, the presence of large homogeneous crystals in nature is an infrequent phenomenon. However, fine-crystalline substances are very common. So, for example, almost all rocks: granite, sandstones, limestone are crystalline. As research methods improved, substances that had previously been considered amorphous turned out to be crystalline. Now we know that even some parts of the body are crystalline, for example, the cornea of the eye, vitamins, the melamine sheath of nerves are crystals. The long path of searches and discoveries, from measuring the external shape of crystals in depth, to the subtleties of their atomic structure is not yet complete. But now researchers have studied its structure quite well and are learning to manipulate the properties of crystals.
Crystals are beautiful, one might say some kind of miracle, they attract to themselves; they say the same "man of a crystal soul" about who has a pure soul. Crystalline means shining with light like a diamond ... And if we talk about crystals with a philosophical attitude, then we can say that this is a material that is an intermediate link between living and inanimate matter. Crystals can be born, aged, destroyed. A crystal, when it grows on a seed (on an embryo), inherits the defects of this very embryo. In general, many examples can be cited that tune in such a philosophical mood, although of course there is a lot of evil ... For example, on television you can now hear about the direct connection of the degree of ordering of water molecules with words, with music, and that water changes depending on thoughts, from the state of health of the observer. Crystals have found their application in various fields: for the manufacture of jewelry, in technology, for example, a ruby laser, liquid crystal screens, etc.
Diamond
About 80% of all mined natural diamonds and all artificial diamonds are used in the Industry.Diamond tools are used to machine parts made from the hardest materials, to drill wells in exploration and mining, serve as reference stones in top-class marine chronometers and other highly accurate instruments. The diamond bearings show no wear even after 25 million revolutions. The high thermal conductivity of diamond allows it to be used as a heat sink substrate in semiconductor electronic microcircuits. Of course, diamonds are also used in jewelry - these are diamonds.
Ruby

The high hardness of rubies, or corundums, has led to their widespread use in industry. From 1 kg of synthetic ruby, about 40,000 watch stones are obtained. The ruby yarn-guides in factories for the production of chemical fibers turned out to be indispensable. They practically do not wear out, while yarn guides made of the hardest glass wear out in a few days when artificial fibers are pulled through them.
New perspectives for the widespread use of rubies in scientific research and technology were opened with the invention of the ruby laser, in which a ruby rod serves as a powerful source of light emitted in the form of a thin beam.
Liquid crystals
These are unusual substances that combine the properties of a crystalline solid and a liquid. Like liquids, they are fluid, like crystals, they have anisotropy. The structure of liquid crystal molecules is such that the ends of the molecules interact very weakly with each other, while the side surfaces interact very strongly and can firmly hold the molecules in a single ensemble. Liquid crystals are used in various kinds of controlled screens, optical shutters, flat-panel television screens.
Laser
The practical part. Stages of work on the project.
| The content of the work at the stage | Teacher activity | Student activities |
| Experimenting | ||
|
Observes, advises, indirectly manages the activities, organizes and coordinates, if necessary, the individual stages of the project. |
|
| Analysis of the data obtained and summing up | ||
| Analysis of the data obtained and summing up | Correction of the conclusions of the project participants during the analysis of the data obtained. |
|
Application
Crystals grown in the course of research work.
These crystals were grown by us in January - May 2010.



We continue our research.
Bibliography:
- Chemistry textbook for university applicants.-ed. Moscow University, 1985
- Shaskolskaya M.P. Crystals .- M.: Science. Main edition of physical and mathematical literature, 1985.-208s.
- Experiments in a home laboratory.- M .: Science. Main edition of physical and mathematical literature, 1980, 144p.
- Myakishev G.Ya. Physics: Molecular Physics. Thermodynamics. 10th grade: Textbook for advanced study of physics. - 5th ed. - M .: Bustard, 2002 .-- 352 p.: Ill.
- Kvant: popular scientific physics and mathematics journal. M .: Science. 1974 year
- Project activities of students. Auth.-comp. N.V. Shirshina. - Volgograd: teacher, 2007 .-- 184 p.
- Lectures in general chemistry. L.S. Guzei: Moscow "First September"
- The world of chemistry. Amusing stories about chemistry. St. Petersburg. "Mim-Express"
GROWING CRYSTALS
IN HOME CONDITIONS
Crystal, as a mysterious and beautiful part of nature, has attracted people's attention since ancient times.
The crystal usually serves as a symbol of inanimate nature. However, the line between living and non-living is very difficult to establish, and the concept of "crystal" and "life" are not mutually exclusive.
Natural crystals have always piqued people's curiosity. Their color, brilliance and shape affected the human sense of beauty, and people decorated themselves and their homes with them. Superstition has long been associated with crystals; as amulets, they were supposed not only to protect their owners from evil spirits, but also to endow them with supernatural powers.
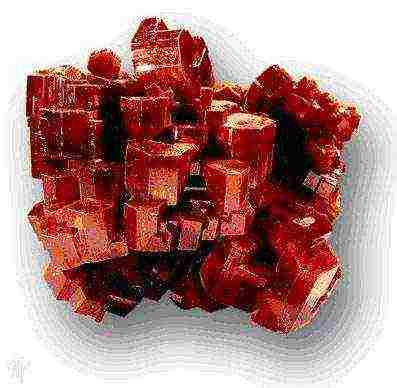
Later, when the same minerals began to be cut and polished like precious stones, many superstitions were preserved in the talismans "for good luck" and "their stones" corresponding to the month of birth. All natural precious stones, except opal, are crystalline, and many of them, such as diamond, ruby, sapphire and emerald, come across as beautifully cut crystals.
The most famous examples of crystals are ice, diamond, quartz, rock salt. Most solids do not have the regular geometric shape of a polyhedron with flat faces and sharp edges characteristic of crystals. The word "crystal" comes from the Greek - "ice".
The nature of crystals
Crystalline substances are solids in which particles (atoms, molecules and ions) are periodically correctly repeated in three dimensions, forming an infinite structure. Particles arranged in space in a certain order form a crystal lattice.
A CRYSTAL LATTICE is a regular arrangement of atoms in space, which determines the specifics of the state of a substance called a solid.
Symmetry and order are the distinguishing characteristics of crystals. Symmetrical bodies are bodies consisting of equal, identical parts that can be combined with each other. There are many different elements of symmetry: plane, axis, center of symmetry, translation, and others.
All crystals are symmetrical. This means that various elements of symmetry can be found in them. Symmetry elements can be combined with each other only according to strict mathematical laws. There can be 230 such combinations for crystal structures in total. They are called "Fedorov space groups" in honor of the crystallographer Fedorov, who, simultaneously with the German mathematician Schoenflis at the end of the 19th century. deduced these laws.
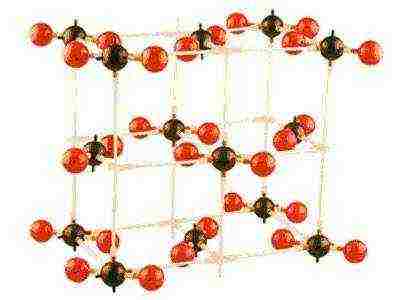
In the crystal lattice, the smallest parallelepiped can be distinguished, when displaced (translated) of which the entire crystal will be obtained in three dimensions. Such a structural unit is called a unit cell. In total, there are 14 elementary three-dimensional geometric cells, or lattices, named after the French scientist who established them, Bravais.
According to the types of chemical bonds, crystals are subdivided into ionic (common table salt), covalent crystals (diamond, silicon), metal, molecular crystals (naphthalene). Different types of bonds in crystals lead to differences in the properties of solids.
How crystals grow.
A crystal is a solid that has a natural polyhedron shape. The chemical bonds of crystals are very ordered and symmetrical. Crystals come in a variety of shapes. Large single crystals with their regular shape are very rare in nature. But such a crystal can be grown in artificial conditions. Crystallization can occur from solution, melt, as well as from the gaseous state of a substance. Consider crystallization from solution.
In a given volume of a particular liquid at a constant temperature and pressure, no more than a certain amount of a particular crystalline substance can dissolve.The resulting solution is called saturated. A crystal placed in a saturated solution will neither grow nor dissolve in it. If you increase the temperature of the liquid, then its solubility
increases, so the available amount of solute will no longer saturate the solution. A crystal placed in an unsaturated solution will start
dissolve in it. If the saturated solution is cooled, it becomes supersaturated. Supersaturated solutions can be stored in closed vessels for a long time without crystallizing. However, it is enough to get into the solution
the smallest particle of crystal, like a solution, will immediately begin to crystallize. Thus, supersaturation of the solution is a necessary but sufficient condition for crystallization. For crystallization to begin
you need to seed the solution - a small crystal of the solute. From a solution, a crystal is usually grown in this way. First, a sufficient amount of crystalline substance is dissolved in water. In this case, the solution is heated until the substance is completely dissolved. Then the solution is slowly cooled, thereby transferring it to a supersaturated state. A seed is added to the supersaturated solution. If, during the entire time of crystallization, the temperature and density of the solution are maintained the same throughout the volume, then during the growth process the crystal will take on the correct shape.
Date Added: 2016-09-06; views: 1456;
Similar articles:


