Content
- 1 Growing organs from stem cells
- 1.1 What are stem cells?
- 1.2 What organs and tissues have scientists been able to grow using stem cells?
- 1.3 In 2005, American scientists grew full-fledged brain cells for the first time.
- 1.4 In 2005, scientists managed to reproduce a neural stem cell
- 1.5 In 2006, Swiss scientists grew human heart valves from stem cells
- 1.6 In 2006, British scientists grew liver tissue from stem cells
- 1.7 In 2006, a complex human organ - the bladder - was grown for the first time in the United States.
- 1.8 American scientists were able to grow a full-fledged bladder in the laboratory. The cells of the patients themselves in need of transplantation were used as material.
- 1.9 In 2007, stem cells helped British scientists create part of the human heart
- 1.10 In 2007, Japanese scientists grew the cornea of the eye from stem cells
- 1.11 In 2007, Japanese scientists grew a tooth from stem cells
- 1.12 In 2008, American scientists were able to grow a new heart on a skeleton from the old
- 2 Growing organs from stem cells
- 3 New way to grow organs for transplant (Video)
- 4 State of the art
- 4.1 Embryoids
- 4.2 Organoids of cardiovascular tissue
- 4.3 Liver organelles
- 4.4 Organoids of the salivary and lacrimal glands
- 4.5 Kidney organelles
- 4.6 Pancreatic organelles
- 4.7 Thymus organelles
- 4.8 Lung tissue organelles
- 4.9 Retinal organelles
- 4.10 Organelles of the sensory epithelium of the inner ear
- 4.11 Organelles of the prostate
- 4.12 Cerebral organelles
- 4.13 Epithelial enteroids, colonoids and cholangioids
- 4.14 Hair follicle spheroids
- 4.15 Bioengineering muscle
- 4.16 Cartilage and muscle tissue for reconstruction operations
- 4.17 Overcoming organ immune rejection
- 4.18 3D bioprinting
- 5 The role of tissue self-organization
- 6 Role of the extracellular matrix
- 7 see also
- 8 Notes (edit)
- 9 Literature
Growing organs from stem cells
Before we move on to the direct story of organ growing, I would like to devote you to what stem cells are.
What are stem cells?

Stem cells - the progenitors of all types of cells in the body, without exception. They are capable of self-renewal and, most importantly, in the process of dividing, they form specialized cells of various tissues. Stem cells renew and replace cells lost as a result of any damage in all organs and tissues. They are designed to restore the human body from the moment of his birth.
With age, the number of stem cells in the body decreases dramatically. In a newborn, 1 stem cell is found in 10 thousand, by the age of 20-25 - 1 in 100 thousand, by 30 - 1 in 300 thousand. By the age of 50, only 1 stem cell per 500 thousand remains in the body. Depletion of stem cells due to aging or serious diseases deprives the body of the ability to heal itself. Because of this, the vital activity of certain organs becomes less effective.
What organs and tissues have scientists been able to grow using stem cells?
I cite only the most famous examples of scientific achievements.
in 2004, Japanese scientists were the first in the world to grow structurally complete capillary blood vessels from stem cells

Japanese scientists were the first in the world to grow structurally complete capillary blood vessels from human embryonic stem cells. This was reported on March 26, 2004 by the Japanese newspaper Yomiuri.
According to the publication, a group of researchers from the Kyoto University School of Medicine led by Professor Kazuwa Nakao used capillary cells generated from stem cells imported from Australia in 2002. Until now, researchers have been able to regenerate only nerve cells and muscle tissue, which is not enough to "produce" a whole organ. Information from the site
In 2005, American scientists grew full-fledged brain cells for the first time.
Scientists from the University of Florida (USA) were the first in the world to grow fully formed and engrafted brain cells. According to the project manager Bjorn Scheffler, the cells were grown by “copying” the regeneration of brain cells. Scientists now hope to grow cells for transplantation, which could help treat Alzheimer's and Parkinson's. Scheffler noted that scientists have previously managed to grow neurons from stem cells, but it was at the University of Florida that they were able to get complete cells and study the process of their growth from start to finish. Information from the site Gazeta.ru based on materials from the Independent.
In 2005, scientists managed to reproduce a neural stem cell
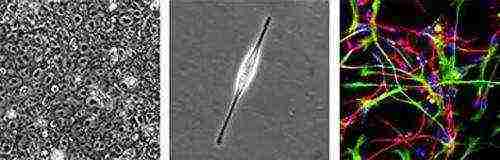
nerve stem cell
An Italian-British group of scientists from the Universities of Edinburgh and Milan has learned how to create various types of cells of the nervous system in vitro on the basis of non-specialized embryonic stem cells.
Scientists have applied already developed methods of control of embryonic stem cells to the more specialized neural stem cells they obtained. The results that have been achieved in mouse cells have been replicated in human stem cells. In an interview with the BBC, Stephen Pollard of the University of Edinburgh explained that his colleagues' development would help recreate Parkinson's or Alzheimer's in vitro. This will allow for a better understanding of the mechanism of their occurrence and development, and will also provide pharmacologists with a mini-testing ground for finding suitable treatments. Corresponding negotiations with pharmaceutical companies are already underway.
In 2006, Swiss scientists grew human heart valves from stem cells
In the fall of 2006, Dr. Simon Hoerstrap and his colleagues at the University of Zurich grew human heart valves for the first time using stem cells taken from amniotic fluid.
This achievement could make it possible to grow heart valves specifically for the unborn child if heart defects are found in the womb. And soon after birth, the baby can be transplanted with new valves.
Following the cultivation of the bladder and blood vessels from human cells in the laboratory, this is the next step towards creating “own” organs for a particular patient, capable of eliminating the need for donor organs or artificial mechanisms.
In 2006, British scientists grew liver tissue from stem cells
In the fall of 2006, British scientists from the University of Newcastle announced that they were the first in the world to grow an artificial liver in laboratory conditions from stem cells taken from umbilical cord blood. The technique used to create the 2cm mini-liver will be further developed to create a normally functioning liver of standard size.
In 2006, a complex human organ - the bladder - was grown for the first time in the United States.

American scientists were able to grow a full-fledged bladder in the laboratory. The cells of the patients themselves in need of transplantation were used as material.
“With a biopsy, you can take a piece of tissue, and after two months its amount will multiply several times,” explains the director of the Institute of Regenerative Medicine Anthony Atala. “We put the starting material and special substances in a special form, leave it in a special laboratory incubator and in a few weeks we get a ready-made organ that can already be transplanted.” The first transplant was performed back in the late 90s. Bladder transplant surgery was performed on seven patients. The results met the expectations of scientists, and now experts are developing methods for creating 20 more organs - among them the heart, liver, blood vessels and pancreas.
In 2007, stem cells helped British scientists create part of the human heart
In the spring of 2007, a group of British scientists, consisting of physicists, biologists, engineers, pharmacologists, cytologists and experienced clinicians, led by Professor of Cardiac Surgery Magdi Yakub, for the first time in history, managed to recreate one of the types of human heart tissue using bone marrow stem cells. This tissue acts as heart valves. If further tests are successful, the developed technique can be used to grow a full-fledged heart from stem cells for transplantation to patients.
In 2007, Japanese scientists grew the cornea of the eye from stem cells
In the spring of 2007, at a symposium on reproductive medicine in the city of Yokohama, the results of a unique experiment by specialists from the University of Tokyo were announced. The researchers used a stem cell taken from the edge of the cornea. Such cells are able to develop into various tissues, performing restorative functions in the body. The isolated cell was placed in a nutrient medium. A week later, it developed into a group of cells, and in the fourth week it was transformed into a cornea with a diameter of 2 cm. In the same way, a thin protective layer (conjunctiva) was obtained, covering the cornea from the outside.
Scientists emphasize that for the first time a full-fledged human tissue has been grown from a single cell. Transplantation of organs obtained by a new method eliminates the risk of transmitting infections. Japanese scientists intend to begin clinical trials immediately after they are convinced of the safety of the new technology.
In 2007, Japanese scientists grew a tooth from stem cells
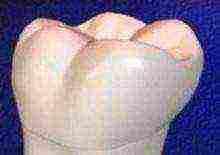
Japanese scientists have managed to grow a tooth from one cell. It was grown in a laboratory and transplanted into mice. The cell material was injected into the collagen scaffold. After cultivation, it turned out that the tooth took on a mature form, which consisted of complete parts such as dentin, pulp, vessels, periodontal tissues, and enamel. According to the researchers, the tooth was identical to the natural tooth. After the transplantation of a laboratory mouse tooth, it engrafted and functioned completely normally. The technique will allow whole organs to be grown from one or two cells, the researchers say.
In 2008, American scientists were able to grow a new heart on a skeleton from the old
Doris Taylor and her colleagues at the University of Minnesota created a living rat heart using an unusual technique. Scientists took an adult rat heart and placed it in a special solution that removed all the cells of the heart muscle tissue from the heart, leaving other tissues intact. This purified scaffold was seeded with heart muscle cells from a newborn rat and placed in an environment that mimics the conditions in the body.
After just four days, the cells multiplied so much that new tissue began to contract, and after eight days, the reconstructed heart was already able to pump blood, albeit at only a 2 percent power level (counting from a healthy adult heart). Thus, the scientists obtained a workable organ from the cells of the second animal. In this way, in the future, it would be possible to process hearts taken for transplantation in order to exclude organ rejection. “You can do any organ like this: kidney, liver, lung, pancreas,” says Taylor. The donor scaffold, which determines the shape and structure of the organ, will be filled with specialized cells made from stem cells that are native to the patient.
It is curious that in the case of a heart, as a basis, you can try to take a pig's heart, anatomically close to a human. By removing only muscle tissue, other tissues of such an organ can already be supplemented with cultured human heart muscle cells, obtaining a hybrid organ, which, in theory, should take root well. And the new cells will immediately be well supplied with oxygen - thanks to the old vessels and capillaries left over from the donor's heart.
Killere 01/28/2009

Medical scientist at work
For many years, scientists around the world have been working to create working tissues and organs from cells. The most common practice is the cultivation of new tissues from stem cells. This technology has been tested for many years and has been consistently successful.But it is not yet possible to fully provide the required number of organs, since it is possible to grow an organ for a particular patient only from his stem cells.
Scientists from Great Britain have succeeded in what no one has been able to do so far - to reprogram the cages and grow a working organ out of them. This will allow in the foreseeable future to provide organs for transplantation to everyone who will need it.
Growing organs from stem cells
Growing organs from stem cells has been familiar to physicians for a long time. Stem cells are the progenitors of all cells in the body. They can replace any damaged cells and are intended to restore the body. The maximum number of these cells occurs in children after birth, and their number decreases with age. Therefore, gradually the body's ability to heal itself decreases.
 Making organs from cells is a complex and expensive process
Making organs from cells is a complex and expensive process
Many fully functioning organs from stem cells have already been created in the world, for example, in 2004, in Japan, capillaries and blood vessels were created from them. And in 2005, American scientists managed to create brain cells. In 2006, human heart valves from stem cells were created in Switzerland. In the same 2006, liver tissue was created in Britain. Until now, scientists have dealt with almost all body tissues, even grown teeth.
A very curious experiment was carried out in the USA - a new heart was grown there on a frame from the old one. The donor heart was cleared of muscles and new muscles were built from stem cells. This completely excludes the possibility of rejection of the donor organ, as it becomes "his". By the way, there are suggestions that as a frame, it will be possible to use a pig's heart, which is anatomically very similar to a human.
A new way to grow organs for transplant (Video)
The main disadvantage of the existing method of growing organs is the need for their production of the patient's own stem cells. Not every patient can take stem cells, and even more so, not everyone has ready-made frozen cells. But recently andResearchers at the University of Edinburgh have been able to reprogram the body's cells so that they can grow the necessary organs from them. According to forecasts, widespread use of this technology will become possible in about 10 years.
To date, scientists have already managed to create a fully functioning thymus gland, which regulates the immune system and is located next to the heart. This organ was made from cells of millet connective tissue, which was obtained from a mouse embryo. The connective tissue cells were transplanted into another cell culture thanks to a special "genetic switch" in the DNA.
Until now, experiments on growing organs in this way have not yielded tangible results. This is the first successful experiment that has shown that it is possible to grow the desired organ even without the use of stem cells, and with the help of any other cells of the body, for example, cells of connective tissues.
Hello! I am Alice. What can I say about myself? Adherent of a healthy lifestyle.
Growing organs - a promising bioengineering technology, the purpose of which is to create various full-fledged viable biological organs for humans. Currently, the technology is not used in humans, since all attempts to transplant such organs have been unsuccessful, but there are active developments and experiments in this area. Using three-dimensional cell cultures, scientists have learned to grow the "rudiments" of organs called organelles (English... organoid, not to be confused with organelles).Such organelles are used by scientists to study and model organogenesis, model tumors and various diseases that can affect certain organs, test and screen various drugs and toxic substances on organoids, as well as for experiments on replacing organs or treating damaged organs with transplants.
State of the art
The idea of artificially growing human organs appeared in the middle of the 20th century, from the moment donor organs began to be transplanted into humans. Even with the possibility of transplanting most of the organs to patients, the issue of donation is currently very acute. A large number of patients die without waiting for their organ. Artificial organ growing, in theory, could save millions of human lives. Some advances in this direction have already been achieved with the help of regenerative medicine methods.
Embryoids
Embryoids or embryonic bodies are three-dimensional aggregates of cells, where cells of all three germ layers are presented, which are necessary for the formation of organs and tissues of the body. Under laboratory conditions, they can be obtained by various cultivation methods from undifferentiated iPSCs. Embryonic body formation is a common method used to differentiate iPSCs into various cell lines.
Organoids of cardiovascular tissue
By cultivating embryoids on collagen-conjugated hydrogels with a stiffness similar to that of cardiac muscle tissue, Shkumatov et al. managed to obtain cardiovascular organelles capable of contraction. Thus, they showed that the rigidity of the extracellular matrix can play an important role in cell differentiation. The need to create mechanical stresses that are comfortable for cultured cells by regulating the rigidity of the substrate material for cultivation was noted in a number of other works. New technologies have made it possible to synchronize the contractions of the cells of the heart organoid. A correctly selected pace of electrical stimulation, forcing the growing muscle tissue to contract, allows not only to shorten the cultivation time, but also to copy mature healthy cardiac tissue in a number of parameters more qualitatively.
Liver organelles
Researchers from Japan have taken an important step towards growing organs in the laboratory. They managed to create a simple but fully functional human liver. The researchers obtained liver cells from iPSCs and cultured them together with endothelial cells (precursors of blood vessels) and mesenchymal cells, which act as the "glue" that unites different cells. It turned out that at a certain ratio of these cells, their joint culture exhibits the ability to self-organize and forms three-dimensional spherical structures that represent the rudiment of the liver. When these liver buds were transplanted into mice, it was found that, in about 48 hours, they form bonds with nearby blood vessels and are able to perform functions characteristic of the liver. According to some scientists, such rudiments of the liver, if reduced in size and then introduced into the bloodstream of the damaged liver, could help normalize its function. Unfortunately, there is still no guarantee that the liver cells derived from iPSCs will not induce the formation of tumors. Careful refinement of these methods is required. On the basis of liver organelles, a device has been created - a bio-artificial liver with liver organelles for the temporary maintenance of the life of patients.
Takebe et al. created a reproducible method for large-scale cultivation of vascularized human liver organelles entirely from induced pluripotent stem cells (iPSCs) and demonstrated their functionality for use as a transplant for the treatment of humans.
Organoids of the salivary and lacrimal glands
A team of researchers from Tokyo University of Science and Corporation Organ Technologies Inc led by Professor Takashi Tsuji (Takashi tsuji) demonstrated functional regeneration of the submandibular salivary glands from bioengineered embryos of the salivary gland after orthotopic (with removal of the defective gland) transplantation, with the aim of restorative therapy by replacing the organ in mice in which the defect of the salivary glands was modeled. The created bioengineered embryo developed into a mature gland through the formation of uviform processes with muscle epithelium and innervation. It produced and secreted saliva in response to taste stimulation with citrate, restored the process of swallowing food, and protected the oral cavity from bacterial infection. The same group successfully performed orthotopic transplantation of bioengineered embryos of the lacrimal glands into mice with a model simulating damage to the corneal epithelium caused by dysfunction of the lacrimal gland. In vivo, bioengineered embryos gave rise to lacrimal glands capable of performing physiological functions, including tear production in response to nerve stimulation, and protection of the ocular surface.
Kidney organelles
Technologies have been developed for growing kidney organelles from pluripotent cells, which can be used to simulate kidney diseases and screen drugs for their treatment, and in the future for implanting miniature kidneys in patients created from their own iPSCs. A strategy has been developed for the transplantation of such an organoid, which allows it to drain the urine secreted by it into the bladder.
Pancreatic organelles
Researchers at the Danish Stem Cell Center have developed a three-dimensional (3-D) gel culture Matrigel with a specially selected composition of the medium, which can be used to grow miniature pancreas "seeds". In the long term, such "frameworks" can be useful for the fight against diabetes as "spare parts".
Thymus organelles
The thymus plays an important role in the generation of new T cells. This gland is very active early in life, but dies off upon reaching adulthood in a process known as thymic involution, resulting in decreased immunity in the elderly. Injecting thymus organelles into the body of old people could help them fight a number of senile diseases. Experiments on growing thymus organoids and their transplantation into athymic mice give hope in this regard. It turned out that thymus organelles are not only able to take root, but can also effectively contribute to the restoration of thymic function in its recipients. In the future, thymus organoids will make it possible to produce modified T-cells in bioreactors for the targeted fight against cancer.
Lung tissue organelles
By acting on the signaling pathways of human iPSCs, it was possible to obtain organelles of the human lungs consisting of epithelial and mesenchymal lung compartments, with structural features characteristic of lung tissues. A modification of this method makes it possible to grow lung tissue organelles in a bioreactor and use them to study lung diseases.
Retinal organelles
Developed 3-D organelles of the eyeball and retina with photoreceptor cells: rods and cones. This will allow in the future to develop methods of treating eye diseases such as retinal degeneration.
Organelles of the sensory epithelium of the inner ear
A similar technology has been used to develop methods for obtaining organelles in the sensory epithelium of the inner ear, which in the future will help combat deafness.
Organelles of the prostate
Organelles of the prostate were obtained by directed differentiation of ESCs. It is noted that the time of exposure to the factors WNT10B / Fgf10, which play a key role in the formation of the prostate, as well as during intrauterine development, is of decisive importance for the formation of epithelial cells of the prostate.
Cerebral organelles
For the purpose of modeling and research in vitro of the human brain and its diseases, a three-dimensional culture of organelles of brain cells obtained from pluripotent stem cells was created. Cerebral organelles (English... Cerebral organoid) can be used to study neurulation and other processes of neurogenesis, as simple models of complex brain tissue to study the effects of toxins and drugs on brain tissue through their safe and cost-effective initial screening, as well as to obtain samples for xenotransplantation.
Epithelial enteroids, colonoids and cholangioids
When modeling epithelial organs, the problem is the variety of sources of epithelial tissues, the extreme sensitivity of the proliferative activity of epithelial cells to external changes, as well as the features associated with the epithelial-mesenchymal transition, which are characteristic exclusively of epithelial tissues. Since the shape of such tissues is mainly a wall, its restoration is associated with a multilayer organization and functionality (peristalsis, nervous regulation). These features of tissue morphology summarize biological problems arising in the search for new effective methods of restorative and regenerative surgery of the walls of hollow epithelial organs (esophagus, stomach, intestines), as well as tubular structures (bile duct, ureter). Organelles obtained from epithelial cells of the small and large intestines will help the study of the human intestine. They can be used to study intestinal stem cells and mechanisms of impairment of physiological functions of the gastrointestinal tract, as well as create tumor organelles for studying cancer and screening drugs.
Hair follicle spheroids
The technique of growing cells in the form of spheroids in a hanging drop has been used to cultivate cells of the papillary layer of human hair follicles. It has been shown that when these cells are grown in the form of spheroids, when the cells grow in a more natural three-dimensional environment and interact with each other, they are able to re-induce the formation of hair follicles in human skin.
Bioengineering muscle
A so-called "muscle" tissue has been created that responds to signals from the nerve thanks to the neuromuscular junction grown from muscle cells and neuronal cells. This tissue can potentially be used for pharmacokinetic analyzes and for creating a drive for the muscles of biorobots and prostheses. Moreover, grown in vitro The bioengineered muscle turned out to be capable of development, regeneration and was able to take root after its transplantation into an animal. A technology has been developed for obtaining muscles from iPSCs, which can be multiplied indefinitely by cultivation, which will make it possible to grow muscle tissue in large quantities
Cartilage and muscle tissue for reconstruction operations
From a small number of cells in the patients' nasal septum, it was possible to grow cartilaginous tissue, which was used for the reconstruction of the nose after removal of the tumor. After more than one year, all patients were satisfied with the aesthetic and functional results of the operation and no negative effects were recorded.
Tissue implants grown in the laboratory from the own muscle and epithelial cells of female patients who needed surgery to reconstruct the vagina, after plastic surgery, not only successfully took root and functioned.
A substrate and a special incubator for growing the human esophagus from patient cells have been created. In the long term, this development will save the life of newborns born without a significant part of the esophagus.
Overcoming organ immune rejection
An important obstacle in the transplantation of tissues and organs is their rejection. Even if the allograft is successful, the organ transplant patient usually has to take anti-rejection drugs for the rest of his life.To make the graft "invisible" to the human immune system, a culture of human embryonic stem cells has been created that synthesize two molecules that suppress the activity of T cells, namely CTLA4-Ig (Cytotoxic T lymphocyte-associated antigen-4-immunoglobulin) and PD-L1 (Programmed death ligand 1), both before and after differentiation. A feature of these cells is that the allogeneic (from another person) tissues formed from them do not cause an immune reaction and rejection after transplantation. This means that the transplantation of organs and tissues grown from these "universal" cells may be possible to carry out without the need to check for compatibility.
3D bioprinting
3D Bioprinting Solutions was the first in the world to create a functioning mouse thyroid gland using 3D bioprinting. The Russian bioprinter FABION was used to print the thyroid gland from cells taken from mice. The printed organs were transplanted into mice whose thyroid gland was destroyed with radioactive iodine. The results of the work were presented by the authors at various scientific conferences and published in peer-reviewed journals for specialists.
The role of tissue self-organization
See also Synthetic morphogenesis
Scientists still cannot explain how cells self-organize into complex tissues. Ordered structures arise from cells without external forces or influence. Throughout development, cells influence each other's behavior and make decisions based on "conversation" with neighbors. According to a Japanese scientist Sasai“Such phenomena of self-organization can only be seen in groups of approximately 1,000 to 100,000 cells. At this level, cells can be directly democratic; they do not need a special governor or president to organize them. " Cells are "sorted": the same type stick together, while the different types remain disconnected. Later, centers of organization arise that guide morphogenesis by isolating growth factors (morphogens) using gradients, the concentrations of which create the so-called biofields. An example of the practical application of concentration gradients is the induced growth of axons along the concentration gradients of specific cytokines.
The process of self-organization of cell culture into organelles can be controlled by selecting the necessary components of the 3D environment. It is important to note that the same organelles can be obtained using different media. It is only important to give the correct "starting" signal, and the self-organization mechanism will do the rest.
Role of the extracellular matrix
For normal functioning and renewal of tissue cells in the body, an intercellular matrix is necessary, which creates, maintains and regulates the conditions for their existence in a niche. The extracellular matrix is a multifunctional system that actively participates in a variety of processes associated with the development of the body, often playing the role of a "hint" that guides the differentiation of cells in one direction or another. The components of the matrix can be divided into two conditional groups: structural proteins, such as fibrillar proteins and glycosaminoglycans, and regulatory proteins, including all kinds of growth factors, matrix cell proteins (proteins of the CCN family, IGFBP, decorin and biglycan), enzymes (metalloproteinases) and receptors (integrins). It is not yet possible to recreate such a complex organ system and architecture by artificial means, for example, using 3D bioprinting. However, scientists have developed technologies for obtaining an extracellular matrix from allografts of donor organs by washing them with solutions of detergents, during which the donor cells are removed and only the cell-free matrix remains, which still preserves the architecture (including the network of blood and lymphatic vessels and the matrix of nervous tissue), and also most of the regulatory proteins. Then this matrix is inoculated with the cells of the recipient and placed in a bioreactor, and various technologies of matrix colonization and its cultivation can be used, including combined ones: for example, 3D bioprinting, static and dynamic cultivation.As a result, it is possible to grow an autograft, which consists of the cells of the recipient and, in theory, should not be rejected by his immune system. This technology allows the cell-free matrix obtained from the donor's heart to be populated with cardiomyocytes obtained from the recipient's iPSC and to grow a functioning heart muscle from them in an incubator that supplies them with a nutrient solution, and also reproduces some parameters of the living organism's environment.
A tracheal prosthesis has been developed, which is 95% composed of the patient's tissue, which avoids organ rejection. The skeleton for the prosthesis is bone grown from periosteal tissue. The inner surface of the organ was created from stem cells and the patient's own mucosa. The bioreactor, in which the new trachea matured for six months, was the tissue of the patient's chest wall. As a result of incubation in the prosthesis, its own vascular system was formed.
see also
- Autotransplantation
- Growing teeth
- Growing thymus from iPSCs
- Decellularization
- Growing human organs and tissues in animals
- Synthetic morphogenesis
- 3D bioprinting
Notes (edit)
- ↑ Leading Surgeons Warn Against Media Hype About Tracheal Regeneration. Retrieved July 2, 2017.
- ↑ Cantrell MA, Kuo CJ. (2015). Organoid modeling for cancer precision medicine. Genome Med .; 7 (1): 32. DOI: 10.1186 / s13073-015-0158-y.PMID 25825593
- ↑ Lancaster MA, Knoblich JA. (2014). Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc .; 9 (10): 2329-40. DOI: 10.1038 / nprot.2014.158. PMID
- ↑ Habka D, Mann D, Landes R, Soto-Gutierrez A (2015) Future Economics of Liver Transplantation: A 20-Year Cost Modeling Forecast and the Prospect of Bioengineering Autologous Liver Grafts. PLoS ONE 10 (7): e0131764. doi: 10.1371 / journal.pone.0131764
- ↑ Steven D. Sheridan, Vasudha Surampudi, Raj R. Rao, (2012). Analysis of Embryoid Bodies Derived from Human Induced Pluripotent Stem Cells as a Means to Assess Pluripotency, Stem Cells International, 2012, Article ID 738910,
- ↑ Toni-Marie Achilli, Julia Meyer, Jeffrey R Morgan, (2012). Advances in the formation, use and understanding of multi-cellular spheroids, Expert Opinion on Biological Therapy, 12 (10), 1347-1360 DOI: 10.1517 / 14712598.2012.707181
- ↑ Carpenedo RL, Sargent CY, McDevitt TC (2007) Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells 25: 2224-2234. DOI: 10.1634 / stemcells.2006-0523
- ↑ Shkumatov A, Baek K, Kong H (2014) Matrix Rigidity-Modulated Cardiovascular Organoid Formation from Embryoid Bodies. PLoS ONE 9 (4): e94764. Doi: 10.1371 / journal.pone.0094764
- ↑ Heras-Bautista, C. O., Katsen-Globa, A., Schloerer, N. E., Dieluweit, S., El Aziz, O. M. A., Peinkofer, G., ... & Pfannkuche, K. (2014). The influence of physiological matrix conditions on permanent culture of induced pluripotent stem cell-derived cardiomyocytes. Biomaterials, 35 (26), 7374-7385.
- ↑ Qiu, Y., Bayomy, A. F., Gomez, M. V., Bauer, M., Du, P., Yang, Y., ... & Liao, R. (2015). A role for matrix stiffness in the regulation of cardiac side population cell function. American Journal of Physiology-Heart and Circulatory Physiology, 308 (9), H990-H997. DOI: 10.1152 / ajpheart.00935.2014
- ↑ Patel, A. K., Celiz, A. D., Rajamohan, D., Anderson, D. G., Langer, R., Davies, M. C., ... & Denning, C. (2015). A defined synthetic substrate for serum free culture of human stem cell derived cardiomyocytes with improved functional maturity identified using combinatorial materials microarrays. Biomaterials. 61, 257-265. DOI: 10.1016 / j.biomaterials.2015.05.019
- ↑ The tiny beating heart grown from STEM CELLS, Mail Online... Retrieved July 2, 2017.
- ↑ Matters of the heart: Researchers create 3-D beating heart (eng.), ScienceDaily... Retrieved July 2, 2017.
- ↑ Anatoly Glyantsev (2018). For the first time, mature heart tissue was grown from stem cells. "Vesti.Nauka" ()
- ↑ Ronaldson-Bouchard, K., Ma, S. P., Yeager, K., Chen, T., Song, L., Sirabella, D., ... & Vunjak-Novakovic, G. (2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature, 556, 239-243 DOI: 10.1038 / s41586-018-0016-3
- ↑ Takanori Takebe, Keisuke Sekine, Masahiro Enomura, et al. & Hideki Taniguchi (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature DOI: 10.1038 / nature12271
- ↑ Human liver raised in mice
- ↑ Huch, M; Gehart, H; Van Boxtel, R; Hamer, K; Blokzijl, F; Verstegen, M. M .; Ellis, E; Van Wenum, M; Fuchs, S. A .; De Ligt, J; Van De Wetering, M; Sasaki, N; Boers, S. J .; Kemperman, H; De Jonge, J; Ijzermans, J. N .; Nieuwenhuis, E. E .; Hoekstra, R; Strom, S; Vries, R. R .; Van Der Laan, L. J .; Cuppen, E; Clevers, H (2015). Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 160 (1-2): 299-312. DOI: 10.1016 / j.cell.2014.11.050. PMC 4313365. PMID 25533785.
- ↑ Researchers test bioartificial liver device to treat acute liver failure (eng.), ScienceDaily... Retrieved July 2, 2017.
- ↑ Takebe T. et al. & Taniguchi H. (2017). Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Reports, 21 (10), 2661-2670. DOI: 10.1016 / j.celrep.2017.11.005
- ↑ Ogawa, M., Oshima, M., Imamura, A., et al. & Tsuji, T. (2013) Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nature Communications; 4, Article number: 2498 DOI: 10.1038 / ncomms3498
- ↑ Hirayama, M., Ogawa, M., Oshima, M., et al. & Tsuji, T. (2013) Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nature Communications, 4, Article number: 2497 DOI: 10.1038 / ncomms3497
- ↑ Little, M. H., & Takasato, M. (2015). Generating a self-organizing kidney from pluripotent cells. Current opinion in organ transplantation, 20 (2), 178-186. DOI: 10.1097 / MOT.0000000000000174
- ↑ Minoru Takasato, Pei X. Er, Han S. Chiu, et al., & Melissa H. Little (2015). Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature, DOI: 10.1038 / nature15695
- ↑ Yokote, S., Matsunari, H., Iwai, S., Yamanaka, S., Uchikura, A., Fujimoto, E., ... & Yokoo, T. (2015). Urine excretion strategy for stem cell-generated embryonic kidneys. Proceedings of the National Academy of Sciences, 201507803. DOI: 10.1073 / pnas.1507803112
- ↑ Greggio, C., De Franceschi, F., Figueiredo-Larsen, M., Gobaa, S., Ranga, A., Semb, H.,… & Grapin-Botton, A. (2013) Artificial three-dimensional niches deconstruct pancreas development in vitro. Development, 140 (21), 4452-4462. doi: 10.1242 / dev.096628
- ↑ Fan, Y., Tajima, A., Goh, S. K., Geng, X., Gualtierotti, G., Grupillo, M., ... & Trucco, M. (2015). Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Molecular Therapy. DOI: 10.1038 / mt.2015.77
- ↑ Artificial thymus can produce cancer-fighting T cells from blood stem cells. Retrieved July 2, 2017.
- ↑ Christopher S Seet, et al., & Amélie Montel-Hagen (2017). Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nature Methods DOI: 10.1038 / nmeth.4237
- ↑ Dye, B. R., Hill, D. R., Ferguson, M. A., Tsai, Y. H., Nagy, M. S., Dyal, R., ... & Spence, J. R. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. Elife, 4, e05098. DOI:
- ↑ Dan C. Wilkinson, Jackelyn A. Alva-Ornelas, Jennifer M.S. Sucre et al., & Brigitte N. Gomperts (2016). Development of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling. Stem Cells Trans Med. DOI: 10.5966 / sctm.2016-0192
- ↑ Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., ... & Sasai, Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature, 472 (7341), 51-56.
- ↑ 3-D ‘mini-retinas’ grown from mouse and human stem cells (eng.), ScienceDaily... Retrieved July 2, 2017.
- ↑ Manuela Völkner et al. & Mike O. Karl (2016). Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Reports DOI:
- ↑ Longworth-Mills, E., Koehler, K. R., & Hashino, E. (2015). Generating Inner Ear Organoids from Mouse Embryonic Stem Cells. Methods in Molecular Biology, 10, 7651 DOI: 10.1007 / 7651_2015_215
- ↑ Calderon-Gierszal EL, Prins GS (2015) Directed Differentiation of Human Embryonic Stem Cells into Prostate Organoids In Vitro and its Perturbation by Low-Dose Bisphenol A Exposure. PLoS ONE 10 (7): e0133238. Doi: 10.1371 / journal.pone.0133238
- ↑ Lancaster, M. A., Renner, M., Martin, C. A., Wenzel, D., Bicknell, L. S., Hurles, M. E., ... & Knoblich, J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501 (7467), 373-379.
- ↑ Smith, I., Silveirinha, V., Stein, J. L., Torre-Ubieta, L., Farrimond, J. A., Williamson, E. M., & Whalley, B. J. (2015). Human neural stem cell-derived cultures in three-dimensional substrates form spontaneously functional neuronal networks. Journal of tissue engineering and regenerative medicine. DOI: 10.1002 / term.2001.
- ↑ Harris, J., Tomassy, G. S. and Arlotta, P. (2015), Building blocks of the cerebral cortex: from development to the dish. WIREs Dev Biol. doi: 10.1002 / wdev.192
- ↑ Anca M Paşca, Steven A Sloan, Laura E Clarke, Yuan Tian, Christopher D Makinson, Nina Huber, Chul Hoon Kim, Jin-Young Park, Nancy A O'Rourke, Khoa D Nguyen, Stephen J Smith, John R Huguenard, Daniel H Geschwind, Ben A Barres, Sergiu P Paşca (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nature Methods; Doi: 10.1038 / nmeth.3415
- ↑ Rene Anand (2015) Scientists Grow Human Fetal Brain in a Lab Dish from Stem Cells. Scicasts
- ↑ Jurgen Knoblich How to build a brain // In the world of science. - 2017. - No. 3. - P. 40 - 44.
- ↑ Stuart M. Chambers, Jason Tchieu, Lorenz Studer Build-a-Brain // Cell Stem Cell. - 2013-10-03. - T. 13, no. 4. - P. 377–378. - DOI: 10.1016 / j.stem.2013.09.010.
- ↑ Schwartza, M P., Houb, Z, Propson N E. et al. & Thomson JA (2015). Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proceedings of the National Academy of Sciences, DOI: 10.1073 / pnas.1516645112
- ↑ Nicholas C. Zachos, Olga Kovbasnjuk, Jennifer Foulke-Abel, Julie In, Sarah E. Blutt Human Enteroids / Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology // Journal of Biological Chemistry. - 2016-02-19. - Vol. 291, iss. 8. - P. 3759–3766. - ISSN 1083-351X 0021-9258, 1083-351X. - DOI: 10.1074 / jbc.r114.635995.
- ↑ Dyuzheva T.G., Lyundup A.V., Klabukov I.D., Chvalun S.N., Grigoriev T.E., Shepelev A.D., Tenchurin T.Kh., Krasheninnikov M.E., Oganesyan R .IN. Prospects for the creation of a tissue-engineered bile duct // Genes and cells. - 2016. - T. 11, No. 1. - S. 43-47. - ISSN 2313-1829.
- ↑ Mahe, M. M., Sundaram, N., Watson, C. L., Shroyer, N. F., & Helmrath, M. A. (2015). Establishment of Human Epithelial Enteroids and Colonoids from Whole Tissue and Biopsy. Journal of visualized experiments: JoVE, (97). 52483. DOI: 10.3791 / 52483
- ↑ Lukovac, S., & Roeselers, G.(2015). Intestinal Crypt Organoids as Experimental Models. In The Impact of Food Bioactives on Health (pp. 245-253). Springer International Publishing. DOI: 10.1007 / 978-3-319-16104-4_22
- ↑ van de Wetering, M., Francies, H. E., Francis, J. M., Bounova, G., Iorio, F., Pronk, A., ... & Clevers, H. (2015). Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell, 161 (4), 933-945. DOI:
- ↑ Higgins C. A., Chen J. C., Cerise J. E., et al. & Christiano A. M. (2013) Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. PNAS, doi: 10.1073 / pnas.1309970110
- ↑ Muscle-powered bio-bots walk on command (eng.), ScienceDaily... Retrieved July 2, 2017.
- ↑ Madden, L., Juhas, M., Kraus, W. E., Truskey, G. A., & Bursac, N. (2015). Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. eLife. DOI:
- ↑ Morimoto, Y., Kato-Negishi, M., Onoe, H., & Takeuchi, S. (2013). Three-dimensional neuron-muscle constructs with neuromuscular junctions. Biomaterials, 34 (37), 9413-9419.
- ↑ Mark Juhas, George C. Engelmayr, Jr., Andrew N. Fontanella, Gregory M. Palmer, and Nenad Bursac. (March 2014). Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. PNAS, DOI: 10.1073 / pnas.1402723111
- ↑ Kirill Stasevich (April 2014). ARTIFICIAL MUSCLES ARE ABLE FOR SELF-TREATMENT. COMPULENT
- ↑ Claudia Fuoco, Roberto Rizzi, Antonella Biondo, et al., (2015). n vivo generation of a mature and functional artificial skeletal muscle. EMBO Molecular Medicine, DOI: 10.15252 / emmm.201404062
- ↑ Engineers Grow Functioning Human Muscle from Skin Cells
- ↑ Ilario Fulco, Sylvie Miot, Martin D Haug, et al. (2014). Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. The Lancet. DOI: 10.1016 / S0140-6736 (14) 60544-4
- ↑ Atlántida M Raya-Rivera, Diego Esquiliano, Reyna Fierro-Pastrana, et al. & Anthony Atala. (2014). Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. The Lancet; DOI: 10.1016 / S0140-6736 (14) 60542-0
- ↑ Stasevich K. THE VAGINA FROM A TEST TUBE HAS BEEN ATTENDED IN THE HUMAN ORGANISM. COMPULENT
- ↑ Jyothsna Vasudevan, Jyothsna Vasudevan. Human Esophagus Created from Stem Cell-Infused 3D Scaffold. Biotechin.Asia. 25 August 2015. Retrieved July 2, 2017.
- ↑ Zhili Rong, Meiyan Wang, Zheng Hu, et al. &, Xuemei Fu. (2014) An Effective Approach to Prevent Immune Rejection of Human ESC-Derived Allografts. Cell Stem Cell; 14 (1): 121 DOI: 10.1016 / j.stem.2013.11.014
- ↑ Plege-Fleck A, Lieke T, Römermann D, Düvel H, Hundrieser J, Buermann A, Kraus L, Klempnauer J, Schwinzer R. Pig to rat cell transplantation: reduced and antibody responses to xenografts overexpressing PD-L1. Xenotransplantation 2014; 21: 533-542. DOI: 10.1111 / xen.12121
- ↑ The thyroid gland, created using 3D bioprinting, was successfully transplanted into mice (Russian). Retrieved July 2, 2017.
- ↑ Elena A. Bulanova, Elizaveta V. Koudan, Jonathan Degosserie, Charlotte Heymans, Frederico DAS Pereira Bioprinting of a functional vascularized mouse thyroid gland construct (English) // Biofabrication. - 2017. - Vol. 9, iss. 3. - P. 034105. - ISSN 1758-5090. - DOI: 10.1088 / 1758-5090 / aa7fdd.
- ↑ Mosaic, Moheb Costandi -... The Man Who Grew Eyes From Scratch (eng.), Gizmodo... Retrieved July 2, 2017.
- ↑ Bement, W. M., & von Dassow, G. (2014). Single cell pattern formation and transient cytoskeletal arrays. Current opinion in cell biology, 26, 51-59.
- ↑ Ishihara, K., Nguyen, P. A., Wühr, M., Groen, A. C., Field, C. M., & Mitchison, T. J. (2014). Organization of early frog embryos by chemical waves emanating from centrosomes. Philosophical Transactions of the Royal Society B: Biological Sciences, 369 (1650), 20130454.
- ↑ Karus, M., Blaess, S., & Brüstle, O. (2014). Self ‐ organization of neural tissue architectures from pluripotent stem cells. Journal of Comparative Neurology.
- ↑ S.A. Zhivolupov, N.A. Rashidov, I.N. Samartsev, E.V. Yakovlev Modern ideas about the regeneration of nerve fibers in injuries of the peripheral nervous system // Bulletin of the Russian Military Medical Academy. - 2013. - No. 3 (43). - S. 190-198. - ISSN 1682-7392.
- ↑ Greggio, C., De Franceschi, F. and Grapin-Botton, A. (2015), Concise Reviews: In Vitro-Produced Pancreas Organogenesis Models in Three Dimensions: Self-Organization From Few Stem Cells or Progenitors. STEM CELLS, 33: 8-14. DOI: 10.1002 / stem.1828
- ↑ Baranovskiy D.S., Demchenko A.G., Oganesyan R.V., Lebedev G.V., Berseneva D.A., Balyasin M.V., Parshin V.D., Lyundup A.V. Obtaining a cell-free matrix of tracheal cartilage for tissue engineering constructions (Russian) // Bulletin of the Russian Academy of Medical Sciences. - 2017 .-- T. 72, no. 4. - P. 254–260. - ISSN 2414-3545. - DOI: 10.15690 / vramn723.
- ↑ Lyundup A.V., Demchenko A.G., Tenchurin T.Kh., Krasheninnikov M.E., Klabukov I.D., Shepelev A.D., Mamagulashvili V.G., Oganesyan R.V., Orekhov A S., Chvalun S.N., Dyuzheva T.G. Increasing the efficiency of colonization of biodegradable matrices with stromal and epithelial cells during dynamic cultivation // Genes and cells. - 2016. - T. 11, No. 3. - S. 102-107. - ISSN 2313-1829.
- ↑ MGH team develops transplantable bioengineered forelimb in an animal model. Massachusetts General Hospital. Retrieved July 2, 2017.
- ↑ Out on a limb: Pioneering scientists grow monkey arms in the lab. WGNO.11 Aug 2015. Retrieved July 2, 2017.
- ↑ Bernhard J. Jank, Linjie Xiong, Philipp T. Moser et al. & Harald C. Ott (2015). Engineered composite tissue as a bioartificial limb graft. Biomaterials, 61, 246-256 DOI: 10.1016 / j.biomaterials.2015.04.051
- ↑ Functional heart muscle regenerated in decellularized human hearts. Retrieved July 2, 2017.
- ↑ Guyette JP, Charest JM, Mills RW, Jank BJ, Moser PT, Gilpin SE, Gershlak JR, Okamoto T, Gonzalez G, Milan DJ, Gaudette GR, Ott HC. (2015). Bioengineering Human Myocardium on Native Extracellular Matrix. Circ Res .; 118 (1), 56-72. DOI: 10.1161 / CIRCRESAHA.115.306874 PMID 26503464
- ↑ Petersburg doctors installed a bioengineering trachea prosthesis (Russian). Retrieved July 2, 2017.
Literature
- Russian scientists have created a bio-artificial liver. September 3, 2014, 14:39
- Andrey Konstantinov (2014). Heart from the bioreactor "Russian Reporter" No. 19 (347)
- Victoria Sevostyanova (2014) Need a new aorta? Grow it yourself !. SCIENCE AND LIFE, 04
- Kirill Stasevich (2015). How to grow a brain in a test tube. SCIENCE AND LIFE № 6
- Kirill Stasevich (2014). The human stomach was grown in a test tube. SCIENCE AND LIFE № 10
- Kondratenko Julia (2015). Organs from the laboratory.
- Rupert Wingfield-Hayes (2014) Japan wants to grow organs in pigs for BBC people, Ibaraki Prefecture, Japan - Video.
- Akkerman, N., & Defize, L. H. (2017). Dawn of the organoid era. BioEssays. DOI: 10.1002 / bies.201600244 Review article for preliminary acquaintance with the methods of growing organelles and their problems
- Takebe, T., Enomura, M., Yoshizawa, E., Kimura, M., Koike, H., Ueno, Y., ... & Taniguchi, H. (2015). Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell stem cell, 16 (5), 556-565. DOI:
- Yin, X., Mead, B. E., Safaee, H., Langer, R., Karp, J. M., & Levy, O. (2016). Engineering Stem Cell Organoids. Cell stem cell, 18 (1), 25-38. DOI:
- Yunying Liu, Ru Yang, Zuping He and Wei-Qiang Gao (2013) Generation of functional organs from stem cells. Cell Regeneration, 2: 1 doi: 10.1186 / 2045-9769-2-1
- Kelly Rae Chi (2015). Orchestrating Organoids. A guide to crafting tissues in a dish that reprise in vivo organs. The Scientist.
- Cultivation Handbook. and the use of organelles (2016). Organoid culture handbook
- Kan Handa, Kentaro Matsubara, Ken Fukumitsu, Jorge Guzman-Lepe, Alicia Watson, Alejandro Soto-Gutierrez. Assembly of Human Organs from Stem Cells to Study Liver Disease // The American Journal of Pathology. - 2014. - Vol. 184, no. 2. - P. 348-357. - DOI: 10.1016 / 0092-8674 (83) 90040-5.
- Melissa A. Kinney, Tracy A. Hookway, Yun Wang, Todd C. McDevitt (December 2013) Engineering Three-Dimensional Stem Cell Morphogenesis for the Development of Tissue Models and Scalable Regenerative Therapeutics. Annals of Biomedical Engineering. DOI: 10.1007 / s10439-013-0953-9
- Lab grown eyes - Video "How the eyes of a live rabbit were grown".
- Hitomi Matsunari, Hiroshi Nagashima, Masahito Watanabe, et al. and Hiromitsu Nakauchi (2013). Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. PNAS, 110 (12), 4557-4562, doi: 10.1073 / pnas.1222902110
- Feng, W., Dai, Y., Mou, L., Cooper, D. K., Shi, D., & Cai, Z. (2015). The Potential of the Combination of CRISPR / Cas9 and Pluripotent Stem Cells to Provide Human Organs from Chimaeric Pigs. International journal of molecular sciences, 16 (3), 6545-6556. DOI: 10.3390 / ijms16036545
- As a living, beating heart is grown from stem cells. MUST WATCH VIDEO
- Christa Nicole Grant, Garcia Mojica Salvador, Frederic G Sala et al. (2015). Human and Mouse Tissue-Engineered Small Intestine Both Demonstrate Digestive And Absorptive Function. American Journal of Physiology- Gastrointestinal and Liver Physiology, DOI: 10.1152 / ajpgi.00111.2014
- Donghui Zhang and Wei Jiang (2015). From One-Cell to Tissue: Reprogramming, Cell Differentiation and Tissue Engineering. BioScience, doi: 10.1093 / biosci / biv016
- Cassandra Willyard (2015). The boom in mini stomachs, brains, breasts, kidneys and more. Nature 523, 520-522 DOI: 10.1038 / 523520a
- Download application guide: Organoid (organ-like structures that can be formed by 3D cell culture) Growth on BME 2.
- Purwada, A., Jaiswal, M. K., Ahn, H., Nojima, T., Kitamura, D., Gaharwar, A. K., ... & Singh, A. (2015). Ex vivo engineered immune organoids for controlled germinal center reactions.Biomaterials, 63, 24-34. DOI: 10.1016 / j.biomaterials.2015.06.002
- Broutier, L., Andersson-Rolf, A., Hindley, C. J., Boj, S. F., Clevers, H., Koo, B. K., & Huch, M. (2016). Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nature Protocols, 11 (9), 1724-1743. DOI: 10.1038 / nprot.2016.097
- García-Domínguez, X., Vera-Donoso, C. D., García-Valero, L., Vicente, J. S., & Marco-Jimenez, F. (2016). Embryonic organ transplantation: the new era of xenotransplantation. In Frontiers in transplantology. InTech. DOI: 10.5772 / 62400
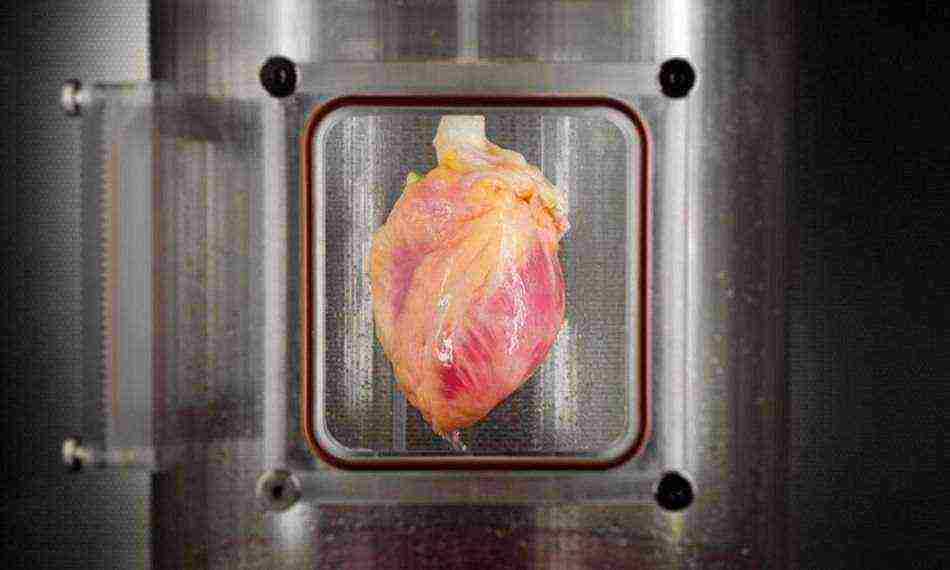
Thousands of people around the world are waiting for donor hearts that can save their lives. But only a few of them get such a chance, and given that the body can reject someone else's organ, this significantly reduces the number of successful transplants. Scientists have long been working to solve this problem, and now a team of researchers from Massachusetts Central Hospital, together with employees of Harvard Medical School, are very close to creating artificially grown hearts.
American scientists have grown human heart tissue in laboratory conditions, as reported in the journal. To create them, the skin cells of an adult were used. Ideally, all this in the future should lead to the cultivation of full-fledged beating hearts from the cells of those people who need an organ transplant. Organs are much easier to grow in a laboratory, where scientists have some kind of scaffolds for future organs through which cells are distributed.
In their previous work, scientists have created a technology that makes it possible to exclude the immune response of the recipient's body when transplanting an organ from another person. They managed to achieve this by removing certain cells potentially capable of causing an immune response from the donor organ using a detergent solution. The scientists repopulated the remaining extracellular matrix with the appropriate type of cells compatible with the recipient. In this way, scientists have already managed to create fully functioning kidneys and lungs for laboratory rats.
The next step of the scientists was to experiment with a real human heart in a specially created bioreactor. The organ was cleared of potentially dangerous cells, after which the remaining scaffold was repopulated with heart cells. The experiments were carried out on 73 human hearts, which were provided to the researchers by one of the organ banks. Don't worry, these hearts were still declared unfit for transplantation, so they could not save anyone's lives.
To obtain heart cells, the researchers used a new method. They reprogrammed adult skin cells using messenger RNA factors, which causes less difficulty in the subsequent regulation of processes. The resulting pluripotent stem cells were differentiated into cardiac muscle cells. The obtained cells were quite enough for research and their transplantation onto cardiac scaffolds. Already a few days later, scientists managed to grow spontaneously contracting muscle tissue on top of the skeleton.
For the first time, scientists have succeeded in regenerating human heart muscle from pluripotent stem cells in the cell-free matrix of an entire human heart. They transplanted about 500 million cells into the wall of the left ventricle of an organ previously devoid of heart cells. After that, the heart stayed in the automated bioreactor system for two weeks. During this time, scientists supplied the heart with a nutrient solution and acted on it with various stress factors. As a result, the cells were transformed into immature heart tissue that responds to electrical stimulation.
Of course, so far all these are just experiments, but, it should be noted, their results are very promising. In the future, such a technology may develop into a full-fledged cultivation of human hearts in vitro, which will be able to give a second chance to those people who have been waiting for a suitable donor organ for years.


